Plus One Chemistry Chapter Wise Questions and Answers Chapter 9 Hydrogen is part of Kerala Plus One Chemistry Chapter Wise Questions and Answers. Here we have given Plus One Chemistry Chapter Wise Questions and Answers Chapter Chapter 9 Hydrogen.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 9 Hydrogen
Plus One Chemistry Hydrogen One Mark Questions and Answers
Question 1.
In which of the following compounds does hydrogen have an oxidation state of -1?
a) CH4
b) NH3
c) HCl
d) CaH2
Answer:
d) CaH2
Question 2.
The radio active isotope of hydrogen is __________ .
Answer:
Tritium
Question 3.
Temporary hardness of water is due to the presence of
a) MgSO4
b) Ca(HCO3)2
c) CaSO4
d) NaHCO3
Answer:
b) Ca(HCO3)2
Question 4.
D2O is used as __________ in nuclear reactors.
Answer:
Moderator
Question 5.
30 volumes of H2O2 means
a) 30% H2O2 solution
b) 30 cm³ of the solution contains 1 g of H2O2
c) 1 cm³ of the solution liberates 30 cm3 of O2 at STP
d) 30 cm³ of the solution contains 1 mole of H2O2
Answer:
c) 1 cm³ of the solution liberates 30 cm3 of O2 at STP
Question 6.
Name the three isotopes of hydrogen.
Answer:
![]()
Question 7.
Dihydrogen is prepared on industrial scale from syngas by
Answer:
Water-gas shift reaction
Question 8.
Among the following elements which do not make a hydride is _________
a) Ti
b) Mg
c) Co
d) Pd
Answer:
c)Co
Question 9.
Hydrogen is purified by _________ .
Answer:
Oculusion on pd
Question 10.
H2O2 is _________ .
Answer:
Diamagnetic
Question 11.
Ortho & para hydrogen are __________ .
Answer:
Nuclear spin isomers
Plus One Chemistry Hydrogen Two Mark Questions and Answers
Question 1.
Write one method each for the laboratory preparation of dihydrogen from
i) mineral acid
ii) aqueous alkali.
- Which is the catalyst used for the reaction?
- Which is the product in this reaction?
Answer:
1.By the reaction of granulated zinc with dilute HCl.
Zn + 2HCl → ZnCl2 + H2
2. By the reaction of zinc with aqueous alkali.
Zn + 2NaOH → Na2ZnO2 + H2
Question 2.
Classify the following into those causing temporary hardness and permanent hardness:
[Mg(HCO3)2, MgCl2, CaCO3, CaSO4, NaCl, NaHCO3, Ca(HCO3)2]
Answer:
Temporary hardness – Mg (HCO3)2, Ca(HCO3)2
Permanent hardness – MgCl2, CaSO4
Question 3.
The bond angle in water is different from the tetrahedral bond angle.
- What is the bond angle and shape of water?
- Justify.
Answer:
1. 104.5°
2. There are three types of repulsions in water. They are: Ip – Ip repulsion, Ip – bp repulsion and bp – bp repulsion. In order to minimize the stronger Ip- Ip repulsion, the bond angle is reduced to 104.5° from 109.5°. Thus, the shape of water is distorted tetrahedral or angular.
Question 4.
Among NH3, H2O and HF which would you expect to have highest magnitude of hydrogen bonding? Why?
Answer:
Strength of H-bond depends upon the atomic size and electronegativity of the other atom to which H- atom is covalently bonded. Smaller size and higher electronegativity favour H-bonding. Among N, F and O atoms, F is the smallest and its electronegativity is highest. Hence, HF will have highest magnitude of H-bonding.
Question 5.
1. Soap does not give lather with hard water. Why?
2. What are the disadvantages of hard water?
Answer:
1. Hard water contains Ca+, Mg2+ions in the form of
their bicarbonates, chlorides or sulphates. Hard water forms scum/precipitate with soap. So soap does not give lather with hard water,
2. Hard water is unsuitable for laundry. It is harmful for boilers because of deposition of salts in the form of scale. This reduces the’ efficiency of the boiler.
Question 6.
Write one example each for the oxidising action of H2O2 in acidic medium and basic medium.
Answer:
In acidic medium, H2O2 oxidises PbS to PbSO4.
PbS(s) + 4H2O2(aq) → PbSO4(s) + 4H2O(l)
In basic medium, H2O2 oxidises Fe2+ to Fe3+.
2Fe2+ + H2O2 → 2Fe3+ + 2OH–
Question 7.
Write one example each for the reducing action of H2O2 in acidic medium and basic medium.
Answer:
In acidic medium H2O2 reduces MnO4– to Mn2+.
2MnO4– + 6H+ + 5H2O2 → 2Mn2+ + 8H2O + 5O2
In basic medium H2O2 reduces l2 to l–.
l2 + H2O2 + 2OH– → 2l– + 2H2O + O2
Question 8.
Write two examples for redox reactions involving water.
Answer:
1. Water can be easily reduced to dihydrogen by highly electropositive metals like Na. Here, Na is oxidised to NaOH.
2H2O(I) + 2Na(s) → 2NaOH(aq) + H2(g)
2. With F2, water is oxidised to O2. Here, F2 is reduced to F-.
2F2(g) + 2H2O(l) → 4H+(aq) + 4F–(aq) + O2(g)
Question 9.
Distinguish between
- Hard and Heavy water
- Temporary and permanent hardness of water.
Answer:
1. A sample of water said to be hard water if it does not give lather with soap. Heavy water is the oxide of deuterium (D2O).
2. Temporary hardness is due to the presence of bicarbonate of calcium or magnesium. It can be removed by boiling.
Permanent hardness is due to the presence of chlorides and sulphates of calcium and magnesium. It is not removed by boiling.
Question 10.
Hydrogen combines with elements to give binary compounds known as hydrides.
1. Name the three categories of hydrides.
2. Classify the given hydrides into different categories: NH3 LiH, TiH, CH4, NaH
Answer:
1. Ionic hydrides, Covalent hydrides and Metallic/ Interstitial hydrides
2. NaH, LiH – Ionic hydrides
NH3, CH4 – Covalent hydrides
TiH – Interstitial hydride
Plus One Chemistry Hydrogen Three Mark Questions and Answers
Question 1.
A chart prepared by a student based on the similarities of hydrogen with alkali metals and halogens is as shown below. Correct the mistakes in it.
| Similarities with alkali metals | Similarities with halogen |
| -1 oxidation state Reducing agent | + 1 oxidation state High Ionisation energy |
| Diatomic state | During electrolysis, both of them are produced at the cathode |
| Non metals |
Answer:
| Similarities with alkali metals | Similarities with halogen |
| +1 oxidation state Reducing agent | -1 oxidation state High Ionisation energy |
| During electrolysis, both of them are produced at the cathode | Diatomic state |
| Non metals |
Question 2.
Prepare a short note on different types of hydrides.
Answer:
Hydrogen reacts with metals or non-metals to form binary compounds known as hydrides.
Hydrides are mainly classified into the following 3 types:
1. Ionic or salt like hydrides
These are stoichiometric compounds of dihydrogen formed with most of the s-block elements which are highly electropositive in character. These are crystalline, non-volatile and non-conducting in solid-state. But their melts conduct electricity and on electrolysis liberate dihydrogen gas at the anode. They react violently with water producing dihydrogen gas. e.g. NaH, KH.
2. Covalent hydrides or Molecular hydrides
These are formed by the action between dihydrogen and nonmetals (p-block elements). Covalent hydrides are classified into electron-deficient (e.g. B2H6), electron-precise (e.g. CH4) and electron-rich hydrides (e.g. NH3).
3. Metallic or Non-stoichiometric or Interstitial hydrides
These are formed by the reaction of dihydrogen with many d-block and f-block elements. These hydrides conduct heat and electricity. They are almost always non-stoichiometric, being deficient in hydrogen, e.g.
LaH2.87,YbH2.55,VH0.56 etc.
Question 3.
Consider the chemical equation and fill in the blanks:
![]()
- This method is used for the preparation of ……………
- The electrode used is …………
- ……….. is produced at cathode.
Answer:
- Hydrogen
- Platinum
- Hydrogen
Question 4.
Analyse the equation: 2Na + H2 → 2NaH
- In this chemical equation, H2 reacts with …………
- Hydrogen reacts with metals to form …………
- Hydrogen is in ………… oxidation state in NaH.
Answer:
- Na
- Metal hydrides
- -1
Question 5.
a) How is hydrogen of high purity prepared?
b) Dihydrogen is relatively inert at room temperature. Give reason.
c) Write any two uses of hydrogen.
Answer:
a) High purity (>99.95%) dihydrogen is obtained by electrolysing warm aqueous barium hydroxide solution bewteen nickel electrodes.
b) This is due to high H-H bond enthalpy.
c) 1. In oxy-hydrogen torches.
2. Liquid hydrogen is used as a fuel in rockets.
Question 6.
A sample of cold river water does not easily give lather with soap, but on boiling it does.
- Evaluate and write the chemical equation involved.
- In some cases, the water does not give ready lather even if it is boiled. Why?
Answer:
1. If the sample of water has temporary hardness, it is removed during boiling. Here, the bicarbonates of magnesium is precipitated as Mg(OH)2 and that of calcium is precipitated as CaCO3.
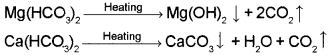
2. The sample of water might have permanent hardness due to the presence of dissolved chlorides and sulphates of Ca and Mg. This cannot be removed just by boiling.
Question 7.
Hydrogen has three isotopes – Protium, Deuterium and Tritium.
a) Of these which is the radioactive one?
b) Name a compound which contains the isotope deuterium.
c) Make a table which shows number of protons, neutrons and electrons in each isotope.
Answer:
a) Tritium
b) Heavy water – D2O
c)
| Isotope | No. of Protons | No. of Neutrons | No. of Electrons |
| Protium | 1 | 0 | 1 |
| Deuterium | 1 | 1 | 1 |
| Tritium | 1 | 2 | 1 |
Question 8.
What are the three types of hydrates? Give examples for each.
Answer:
1. Hydrates with coordinated water, e.g. [Cr(H2O)6]Cl3.
2. Hydrates with interstitial water, e.g. BaCl2.2H2O
3. Hydrates with hydrogen bonded water, e.g. CuSO4.5H2O.
Question 9.
1. What is meant by amphoteric nature of water?
2. Suggest an example to show this property.
Answer:
1. The amphoteric nature of water means that it has the ability to act as an acid as well as a base.
2. Water acts as an acid with NH3 and a base with H2S.
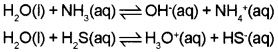
Question 10.
a) What are the advantages of dihydrogen as a fuel?
b) What are the disadvantages of using dihydrogen as fuel?
c) What is meant by the term ‘hydrogen economy’?
Answer:
a) 1. It is abundantly available in the combined state as water.
2. Use of dihydrogen as fuel provides pollution-free atmosphere because its combustion product is only water.
3. Heat of combustion per gram of dihydrogen is more than twice that of jet fuel.
b) 1. Dihydrogen does not occur in free state in nature.
2. Hydrogen gas has explosive flammability which causes problem to its storage and transportation.
3. A cylinder of compressed dihydrogen weighs about 30 times as much as a tank of petrol containing the same amount of energy.
c) Hydrogen economy refers to the use of dihydrogen as an alternative source of energy. The basic principle of hydrogen economy is storage and transportation of energy in the form of dihydrogen instead of fossil fuels or electric power.
Question 11.
What do you mean by temporary and permanent hardness? Explain one chemical method each for removing temporary hardness and permanent hardness.
Answer:
Temporary hardness is due to the presence of bicarbonates of calcium or magnesium. Permanent hardness is due to the presence of chlorides and sulphates of calcium or magnesium ions.
Clark’s method :
This method is used for removing temporary hardness. In this method, calculated amount of lime is added to hard water. It precipitates out calcium carbonate and magnesium hydroxide which can be filtered off.
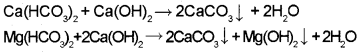
Treatment with washing soda: This method is used to remove permanent hardness. Washing soda reacts with soluble calcium and magnesium chlorides and sulphates in hard water to form insoluble carbonates.
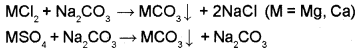
Question 12.
a) Write a method for the preparation of H2O2.
b) What is 100 volume H2O2?
c) Draw the structure of H2O2 in gas phase.
Answer:
a) H2O2 is prepared by acidifying barium peroxide and removing excess water by evaporation under reduced pressure.
BaO2.8H2O(s) + H2SO2(aq) → BaSO4(s) + H2O2(aq) + 8H2O(I)
b) A 30% solution of H2O2 is called 100 volume H2O2. It means that one mL of 30% H2O2 solution will give 100 V of oxygen at STP.
c) H2O2 is had a non-planar structure as shown below:
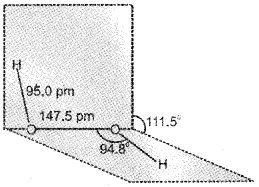
Question 13.
the ion exchange process is commonly employed for large scale production of soft water.
- What is the basic principle involved in the ion-exchange method of softening water?
- What are inorganic cation exchangers? Give example.
Answer:
1. Adsorption
2. These are complex inorganic salts like sodium- aluminium silicate NaAlSiO4 which can exchange cations such as Ca2+ and Mg2+ ions in hard water for Na+ ions. e.g. Zeolite.
Question 14.
1. What is heavy water? Mention one use of heavy water.
2. Explain why hydrogen peroxide is not stored in glass vessels.
3. What is calgon? What is its use?
Answer:
1. D2O. Used as moderator in nuclear reactors.
2. To prevent its decomposition.
3. Calgon is chemically sodium hexametaphosphate (Na6P6O18). It is used to remove Ca2+ and Mg2+ ions of hard water by converting them into soluble complexes.
M2+ + Na4P6O182- → [Na2MP6O18]2- + 2Na+ (M = Mg,Ca)
Question 15.
1. Name the oxide of isotope of hydrogen used in nuclear reactor.
2. What are cation exchange resins? What is their role in removing permanent hardness of water?
Answer:
1. Heavy water (D2O).
2. Cation exchange resins contain large organic molecules with -SO3H group and are water insoluble. Ion exchange resin (RSO3H) is changed to RNa by treating with NaCl. The resin exchanges Na+ ions with Ca2+ and Mg2+ ions present in hard water to make the water soft.
Question 16.
1. What is permuitit? How is it useful in removing permanent hardness of water?
2. Compare the structures of water and hydrogen peroxide.
Answer:
1. Hydrated sodium aluminium silicate is called permutit (NaZ). When it is added to hard water it exchanges Ca2+ and Mg2+ ions with Na+ ions.
2NaZ(s) + M2+(aq) —> MZ2(s) + 2Na+(aq) M = Ma, Ca)
2. Water molecule has an angular or bent shape. H2O2 has a non-planar structure.
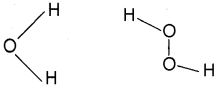
Question 17.
1. Give the industrial method of preparation of H2O2.
2. How is heavy water prepared?
3. How is pure de-mineralized water obtained?
Answer:
1. Industrially H2O2 is prepared by the auto-oxidation of 2-ethylanthraquinol.
2. Heavy water is prepared by exhaustive electrolysis of water or as a byproduct in some fertilizer industries.
3. Pure de-mineralised water is obtained by passing water successively through a cation exchange and an anion exchange resins.
In the cation exchange process, the H+ exchanges for Na+, Ca2+, Mg2+ and other cations present in water. This process results in proton release and makes the water acidic.
2RH(s) + M2+(aq) ⇌ MR2(s) + 2H+(aq)
In the anion exchange process, the OH– exchanges for anions like Cl–, HCO3–, SO42- etc. present in water.
RNH3+OH–(S) + X–(aq) ⇌ RNH3+X–(s) + OH–(aq)
The OH– ions, thus liberated neutralise the H+ ions set free in the cation exchange process to get pure de-mineralised water.
H+(aq) + OH–(aq) → H2O(l)
Question 18.
1. Explain why hydrogen peroxide is stored in coloured plastic bottles.
2. Write any two uses of H2O2.
Answer:
1. In the presence of metal surfaces or traces of alkali (present in glass conatiners), the decomposition of H2O2 (2H2O2 → 2H2O + O2) is catalysed. It is therefore stored in wax-lined glasses or plastic vessels in dark.
2. 1. As a hair bleach and as a mild disinfectant.
2. In the synthesis of hydroquinone, tartaric acid and certain food products and pharmaceuticals.
Question 19.
1. A sample of hard water was found to loose its hardness on boiling. Name the type of hardness associated with this sample. 0
2. Write the name and formula of four minerals which cause permanent hardness of water.
3. What are electron deficient hydrides? Whether they behave as Lewis acids or Lewis bases? Why?
Answer:
1. Temporary hardness
2. CaCl2, MgCl2, CaSO4, MgSO4
3. These are covalent hydrides having too few electrons for writing conventional Lewis structure. They act as Lewis acids because they can accept electrons, e.g. B2H6
Plus One Chemistry Hydrogen Four Mark Questions and Answers
Question 1.
Match the following:
| A | B |
| Temporary hardness | p-block elements |
| Hydrides | Reducing agent |
| Permanent hardness | Chloride |
| Alkali metals | Bicarbonate |
Answer:
| A | B |
| Temporary hardness | Bicarbonate |
| Hydrides | p-block elements |
| Permanent hardness | Chloride |
| Alkali metals | Reducing agent |
Question 2.
Certain samples of water do no produce easy lather with soap.
- What is this condition of water called?
- Which are two types of this condition?
- Suggest two methods to change this condition of water.
- What are the problems caused by this condition of water?
Answer:
- Hardwater.
- Temporary hardness and permanent hardness.
- By boiling water and by adding Na2CO3
- Wastage of soap and boiler explosion.
Question 3.
Match the following:
| 1. D2O | a) Hendry Cavendish |
| 2. Hydrogen | b) Water gas |
| 3. Ca + H2 | c) 31H |
| 4. C2H4 + H2 | d) Heavy water |
| 5. Tritium | e) 21H |
| 6. Deuterium | f) CaH2 |
| 7. CO + H2 | g) C2H6 |
| 8. CO + Z2 | h) Producer gas |
Answer:
1) – d)
2) – a)
3) – f)
4) – g)
5) – c)
6) – e)
7) – b)
8) – h)
Question 4.
1. A list of compounds are given below:
H2O, HCl, CH4
Construct chemical reactions to show the preparation of H2 from each of the above compounds.
2. What is syngas? How is it prepared?
3. What is coal gasification?
4. Explain water gas shift reaction.
Answer:
1.
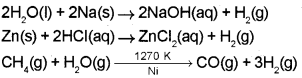
2. Syn gas is a mixture of CO and H2. It is prepared by passing steam over red hot coke.
![]()
3. The process of production of syngas from coal is called coal gasification.
4. The production of dihydrogen can be increased by reacting CO of syngas mixtures with steam in the presence of iron chromate as catalyst. This is called water gas shift reaction.
![]()
The CO2 is removed by scrubbing with sodium arsenite solution.
Plus One Chemistry Hydrogen NCERT Questions and Answers
Question 1.
Write the names of isotopes of hydrogen. What is the mass ratio of these isotopes? (2)
Answer:
The various isotopes of hydrogen are:
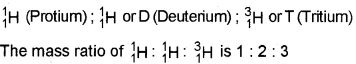
Question 2.
Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions? (2)
Answer:
Hydrogen atom has only one electron and thus, to achieve stable inert gas configuration of helium, it shares its single electron with electron of another hydrogen atom to form a stable diatomic molecule. The stability of H2 is further confirmed by the fact, that formation of one mole of gaseous H2 molecules results in the release of 435.8 kJ of energy.
H(g) + H(g) H2(g); ∆H = – 435.8 kJ mol-1
Question 3.
How do you expect the metallic hydrides to be useful for hydrogen storage? Explain. (2)
Answer:
In some of the transition metal hydrides, hydrogen is absorbed as H-atoms. Due to the inclusion of H- atoms, the metal lattice expands and thus becomes less stable. Therefore, when such metallic hydride is heated, it decomposes to release hydrogen gas and very finely divided metal. The hydrogen evolved in this manner can be used as a fuel. Thus, transition metals or their alloys can act as sponge and can be used to store and transport hydrogen to be used as a fuel.
Question 4.
What causes the temporary and permanent hardness of water? (2)
Answer:
Temporary hardness is caused by presence of bicarbonates of calcium and magnesium, i.e., Ca(HCO3)2 and Mg(HCO3)2 in water whereas permanent hardness is caused by presence of soluble chlorides and sulphates of calcium and magnesium, i.e., CaCl2, CaSO4, MgCl2 and MgSO4 in water.
Question 5.
Write chemical reactions to show amphoteric nature of water. (2)
Answer:
Water is amphoteric in character. It means that it can act as proton donor as well as proton acceptor. When it reacts with acids, (stronger than itself), it behaves as a base. When it reacts with bases (stronger than itself) it acts as an acid.
Question 6.
Is demineralised or distilled water useful for drinking purposes? If not, how can it be made useful? (2)
Answer:
Demineralised or distilled water is not useful for drinking purposes because it does not contain even useful minerals. Therefore, to make it useful for drinking purposes, useful minerals in proper amounts should be added to demineralised or distilled water.
Question 7.
How does H2O2 behave as a bleaching agent? (2)
Answer:
The bleaching action of H2O2 is due to the nascent oxygen which is liberates on decomposition.
H2O2 → H2O + [O]
The nascent oxygen oxidise the colouring matter to colourless products. Hence, H2O2 is used for the bleaching of delicate maerials like ivory, feather, silk, wool, etc.
We hope the Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 9 Hydrogen help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 9 Hydrogen, drop a comment below and we will get back to you at the earliest.
