Plus One Chemistry Chapter Wise Questions and Answers Chapter 4 Chemical Bonding and Molecular Structure is part of Kerala Plus One Chemistry Chapter Wise Questions and Answers. Here we have given Plus One Chemistry Chapter Wise Questions and Answers Chapter 4 Chemical Bonding and Molecular Structure.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 4 Chemical Bonding and Molecular Structure
Plus One Chemistry Chemical Bonding and Molecular Structure One Mark Questions and Answers
Question 1.
The octet rule is not valid for
a) CO2
b)H2O
c) O2
d) CO
Answer:
d) CO
Question 2.
The stability of an ionic crystal depends principally on
a) High electron gain enthalpy of the anion forming species
b) The lattice enthalpy of the crystal
c) Low ionization enthalpy of the cation forming species
d) Low heat of sublimation of cation forming solid
Answer:
b) The lattice enthalpy of the crystal
Question 3.
Which of the following molecules has highest dipole moment?
a) H2S
b)CO2
c) CCl4
d) BF3
Answer:
a) H2S
Question 4.
The d-orbital involved in sp3d hybridization is .
Answer:
dz2
Question 5.
Which of the following is paramagnetic and has a bond order of ½?
![]()
Answer:
H2+
Question 6.
Dipole moment µ electric charge ‘e’ and bond length ‘d’are related by the equation.
Answer:
M = e x d
Question 7.
In which of the following carbon atom is sp2 hybridised?
a) CO2
b) C2H6
c) C6H6
d) HCN
e)
Answer:
C6H6
Question 8.
AgF is ionic whereas Agcl is covalent. This can be explained by
Answer:
Faja’ens Rule
Question 9.
The shape of covalent molecule CIF3 is _________
Answer:
T – shaped
Question 10.
The C – O bond order in CO32- is
Answer:
1.33
Plus One Chemistry Chemical Bonding and Molecular Structure Two Mark Questions and Answers
Question 1.
The order of repulsion of electron pairs as written by student is given below:
lone pair-lone pair repulsion < lone pair-bond pair repulsion>bond pair-bond pair repulsion.
1. Can you see anything wrong in this?
If yes, correct it.
2. Name the theory behind this.
Answer:
1. Yes.
Repulsion decreases in the order: lone pair-lone pair repulsion>lone pair-bond pair repulsion> bond pair-bond pair repulsion,
2. VSEPR theory
Question 2.
During a small group discussion in the class room a student argued that in acetylene both the carbon atoms are in sp3 hybridised state.
- What is your opinion?
- What is the bond angle between carbon atoms in acetylene?
Answer:
- The student’s argument is wrong. In acetylene both the carbon atoms are triple bonded and are in sp hybridised state,
- 180°
Question 3.
Classify the following compounds according to their hybridisation.
CH4, BF3, C2H4, BeF2, C2H2
Answer:
sp3 hybridisation – CH4
sp2 hybridisation – BF3, C2H4
sp hybridisation – BeF2, C2H2
Question 4.
A student arranged the halide ions in the increasing order of polarisability as: F < l < CI < Br
1. Is this the correct order? If not write it in correct order.
2. Justify.
Answer:
1. No.
Polarisability increases in the order: F– < Cl– <Br– < l–
2. Polarisability increases when the size of anion increases.
Question 5.
Give any two differences between sigma and pi bonds.
Answer:
Sigma bond (σ) is formed when two atomic orbitals under head-on overlapping. It is a strong bond. Pi(π) bond is formed when atomic orbitals undergo lateral (sidewise) overlapping. It is a weak bond.
Question 6.
Write the type of hybridisation of each carbon in the compound.
CH3-CH=CH-CN
Answer:
Carbon 1 → sp³
Carbon 2 → sp²
Carbon 3 → sp²
Carbon 4 → sp
Plus One Chemistry Chemical Bonding and Molecular Structure Three Mark Questions and Answers
Question 1.
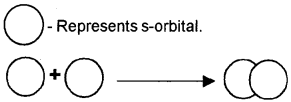
1. What is meant by the above picture?
2. Which type of bond is present here?
3. Which type of overlapping leads to the formation of π bond?
Answer:
- s-s overlapping
- A strong sigma bond
- This type of covalent bond is formed by the lateral or sidewise overlap of half-filled atomic orbitals.
Question 2.
‘In ethane there are 6 covalent bonds. Five are strong σ bonds and the remaining one is a weak π bond.’
- Do you agree with this?
- How is a bond different from π bond in the mode of formation?
Answer:
- Yes.
- Sigma bond is formed by the end to end overlapping of bonding orbitals along the internuclear axis, π bond is formed by the lateral or sidewise overlap of half filled atomic orbitals.
Question 3.
Choose the correct molecules from the given clues: H2O, SF6, BF3
1. Clue -1 The central atom is in sp² hybridised state and the molecule has trigonal planar in shape. Clue-2 The bond angle is 120°
2. Clue-1 The number of electron pairs in this molecule is 6.
Clue -2 It has octahedral geometry.
3. Clue-1 The bond angle is reduced from 109° 28′ to 104.5°
Clue-2 It has a bent shape.
Answer:
- BF3
- SF6
- H2O
Question 4.
Give theoretical explanation for the following statements.
1. H2S is acidic while H2O is neutral.
2. Hydrogen chloride gas dissolves in water.
Answer:
1. S-H bond energy is less than that of O-H bond energy. So H+ can be easily generated from H2S.
2. When HCl is treated with H2O it undergoes hydrolysis as per the following reaction and dissolves.
HCl + H2O → H3O+ +Cl–
Question 5.
The potential energy level diagram forthe formation of hydrogen molecule as drawn by a student is given below:
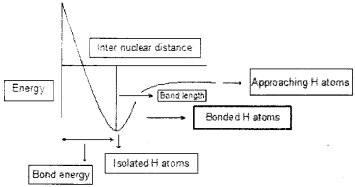
1. Resketch the graph with correct labelling.
2. How can you determine the radius of one hydrogen atom?
Answer:
1.
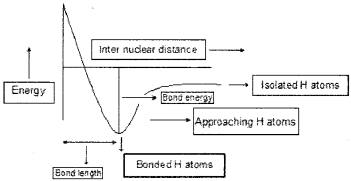
2. Bond length in hydrogen molecule is the intermolecular distance between two hydrogen atoms. So, half of the bond length is taken as the radius of hydrogen atom.
Question 6.
Ionisation enthalpy is one of the factors favoring the
formation of ionic bonds.
1. Will you agree with this statement?
2. Explain how?
3. Write another factor favouring the formation of ionic bonds.
Answer:
1. Yes.
2. In the formation of the ionic bond, a metal atom losses electrons to form cation. This process requires energy equal to the ionisation enthalpy. Lesser the ionisation enthalpy of the metal atom, easier will be the removal of electron from the atom to form cation and hence greater will be the tendency to form ionic bond.
3. Electron gain enthalpy of the element forming anion.
Question 7.
Complete the following table:
| Sigma bond | π -bond | |
| Formation | …………. | …………. |
| Strong/Weak | …………. | …………. |
| About rotation | …………. | …………. |
Answer:
| Sigma bond | π -bond | |
| Formation | Sigma bond is formed by end to end (or axial) overlap of atomic orbitals | π -bond is formed by the sidewise overlap of atomic orbitals |
| Strong/Weak | Strong | Weak |
| About rotation | Free rotation | Free rotation is not possible |
Question 8.
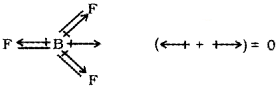
a) The dipole moment of BF3 is zero even though the B – F bonds are polar. Justify.
b) Give the hybridisation involved in the following compounds
- NH3
- C2H4
- SF6
- PCl5
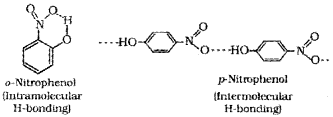
c) o-nitro phenol has a lower boiling point than its para isomer. Why?
Answer:
a) In BF3, dut to the symmetric trigonal planar geometry of the molecule, the B – F bond are oriented at an angle of 120°to one another. The three bond moments give a net sum of zero as the resultant of any two is equal and opposite to the third.
b)
- sp³
- sp²
- sp³d²
- sp³d
c) In o-nitrophenol, intramolecular hydrogen bonding is present and there is no association of molecules whereas in p-nitrophenol there is inter-molecular hydrogen bonding which causes association of molecules.
Question 9.
1. How many a and n bonds are there in the following molecules i) ethane ii) acetylene?
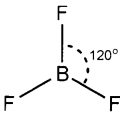
2. BF3 and NH3 are tetra atomic molecules. But the shape of BF3 is different from that of NH3. Explain this using hybridisation.
Answer:
1. i) Ethane – 7σ bonds
ii) Acetylene-3σ bonds and 2 π bonds
2. In BF3 molecule, the ground state electronic configuration of central boron atom is 1s²2s²2p¹. In the excited state, one of the 2s electrons is promoted to vacant 2p orbital. As a result, boron has three unpaired electrons. These three orbitals (one 2s and two 2p) hybridise to form three sp2 hybrid orbitals oriented in a trigonal planar arrangement and overlap with 2 p orbitals of F to form three B – F bonds. Therefore, BF3 molecule has a planar geometry with FBF bond angle of 120°.
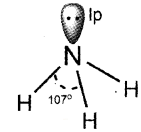
In NH3, the valence shell electronic configuration of N in ground state is 2s² . These four orbitals undergo sp³ hybridisation to form four sp³ hybrid orbitals, three of them containing unpaired electrons and the fourth one containing lone pair. The three hybrid orbitals overlap with 1 s orbitals of hydrogen atoms to form three N – H sigma bonds. Since, the bp-lp repulsion is greater than the bp-bp repulsion, the molecule gets distorted and the bond angle is reduced to 107° from 109.5°. Thus, the geometry of NH3 molecule is trigonal pyramidal.
Question 10.
Covalent bond is formed by the overlaping of atomic orbitals.
1. What is meant by orbital overlapping?
2. What are the 3 types of overlapping?
Answer:
1. Orbital overlapping is the partial interpenetration or merging of atomic orbitals. It results in the pairing of electrons. Greater the overlap the stronger is the bond formed between two atoms.
2. s-s overlapping
s-p overlapping
p-p overlapping
Question 11.
1. Which among the following will exist He2 or He2+? Explain.
2. H2S is a gas at ordinary condition, while H2O is liquid. Account for the above statement.
3. State the hybridisation in the following molecules,
i) PF6
ii) C2H6
Answer:
1. He2+
Helium molecule contains 4 electrons. Out of this 4 electrons, 2 are present in the bonding molecular orbital and the remaining 2 are present in the anti-bonding molecular orbital.
Bond order = ½ (Nb-Na)
= ½ (2-2) = 0
Hence, He2 cannot exist. The molecular orbital diagram is given below:
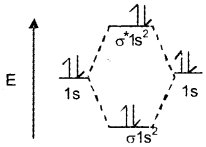
He2+ contains 3 electrons. Out of these 3 electrons, 2 are present in the σ1s level and the remaining one is present in the σ* 1s level.
Bond order = ½ (Nb-Na)
= ½ (2-1) = ½
Since the bond order is half the molecular ion exists but possesses low stability.
2. In H2S, there is no hydrogen bonding whereas in water, molecular association is possible due to intermolecular hydrogen bonding.
3. i) PF5 = sp³d hybridisation
ii) C2H6 = sp³ hybridisation
Question 12.
Bond order is a term commonly used in MO theory.
1. How is it calculated?
2. How is it related to bond length and bond energy?
Answer:
1. Bond order = ½ (Nb-Na)
where Nb = No. of electrons occupying bonding orbitals and Na = No. of electrons occupying antibonding orbitals.
2. As the bond order increases, bond length decreases and bond energy increases, i.e., bond order is directly proportional to bond energy and inversely proportional to bond length.
Question 13.
1. Explain the hybridisation and geometry of ethyne.
2. What is the difference between bonding molecular orbital and antibonding molecular orbital?
3. How the magnetic nature of a molecule is related to its electronic structure?
Answer:
1. In the formation of ethyne (C2H2), both the carbon atoms undergo sp hybridisation having two unhybridised orbitals (2px and 2py). One sp hybrid orbital of one carbon atom overlaps axially with sp hybrid orbital of the other carbon atom to form C-C sigma bond, while the other hybridised orbital of each carbon atom overlaps axially with the half-filled s orbital of hydrogen atoms forming o bonds. Each of the two unbybridised p orbitals of both the carbon atoms overlaps sidewise to form two K bonds between the carbon atoms. Thus, ethyne has a linear geometry with π bond angle of 180°.
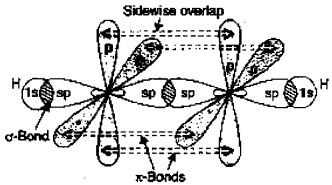
2. The molecular orbital which has lower energy than the atomic orbital is called bonding molecular orbital and the molecular orbital which has greater energy than the atomic orbital is called antibonding molecular orbital.
3. If all the molecular orbitals in a molecule are doubly occupied (i.e., paired), the substance is diamagnetic (repelled by magnetic field). It one or more molecular orbitals are singly occupied (i.e., unpaired) it is paramagnetic (attracted by magnetic field).
Question 14.
Molecular Orbital Theory (MOT) is an advanced theory
of chemical bonding.
1. Write the salient features of MOT.
2. What is meant by LCAO? Illustrate using hydrogen molecule.
3. What are the conditions for the combination of atomic orbitals?
Answer:
1.
- The electrons in a molecule are present in the
various molecular orbitals. - The atomic orbitals of comparable energies and proper symmetry combine to form molecular orbitals.
- The electron in a molecular orbital is influenced by two or more nuclei depending upon the number of atoms in the molecule.
- The number of molecular orbitals formed is equal to the number of combining atomic orbitals. When two atomic orbitals combine, two
molecular orbitals are formed. One is known as bonding molecular orbital while the other is called antibonding molecular orbital. - The bonding molecular orbital has lower energy and hence greater stability than the corresponding antibonding molecular orbital.
- The electron probability distribution around a group of nuclei in a molecule is given by a molecular orbital.
- The molecular orbitals are filled in accordance with the Aufbau principle obeying the Pauli’s exclusion principle and the Hund’s rule.
2. LCAO refers to the linear combination of atomic orbitals. It is an approximate method used to explain the formation of molecular obritals. Consider hydrogen molecule consisting of two atoms A and B. Each hydrogen atom has one electron in the 1s orbital. The atomic orbitals of these atoms can be represented by the wave functions ψA and ψB. Mathematically, the formation of molecular orbitals can take place by addition and by subtraction of wave functions of individual atomic orbitals.
ψMO = ψA ± ψB
Therefore, the two molecular orbitals σ and σ* are formed as:
σ* = ψA – ψB
The molecular orbital σ formed by the addition of atomic orbitals is called the bonding molecular orbital while the molecular orbital σ* formed by the subtraction of atomic orbital is called antibonding molecular orbital. The energy level diagram is shown below:
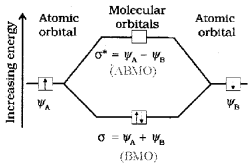
3.
- The combining atomic orbitals must have the same or nearly same energy.
- The combining atomic orbitals must have the same symmetry about the molecular axis.
- The combining atomic orbitals must overlap to the maximum extent.
Question 15.
Consider a reaction PCl5(g) → PC3(g) + Cl2(g)
1. What is the change in hybridisation state of phosphorus?
2. Explain why does PCl5 decomposes easily?
Answer:
1. When PCl5 decomposes to PCl3, the hybridisation of P changes from sp³d to sp³.
2. In PCl5, the five sp³d orbitals of P overlap with the singly occupied p orbitals of Cl atoms to form five P-CI sigma bonds. Three P-Cl bonds which lie in one plane and make an angle of 120° with each other are called equatorial bonds. The remaining two P – Cl bonds, called axial bonds, one lie above and the other lie below the equatorial plane, make an angle of 90° with the plane. As the axial bond pairs suffer more repulsive interaction from the equatorial bond pairs, axial bonds are slightly longer and hence slightly weaker than the equatorial bonds. This makes PCl5 molecule more reactive and hence it decomposes easily.
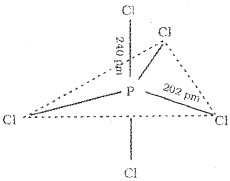
Question 16.
The electron dot structure (Lewis structure) of ammonia molecule is shown below:
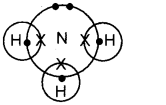
1. Write the number of bond pairs of electrons and lone pairs of electrons in ammonia molecule.
2. The structures of o-nitrophenol and p-nitrophenol are shown in the figure. The former is a steam volatile liquid whereas the latter is a solid. Justify your answer giving reason.
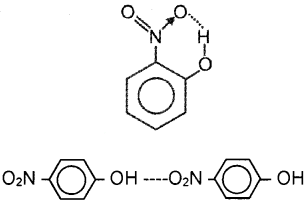
Answer:
1. Ammonia molecule contains one lone pair of electrons and 3 bond pair of electrons,
2. In o-nitrophenol, there is intramolecular hydrogen bonding and there is no molecular association. But in p-nitro phenol intermolecular hydrogen bonding is present and hence molecular association is possible.
Plus One Chemistry Chemical Bonding and Molecular Structure Four Mark Questions and Answers
Question 1.
Hydrogen bonding is present in NH3 and H2O.
1. What is hydrogen bond?
2. What are different types of hydrogen bonds?
3. Explain the effect of hydrogen bonding.
Answer:
1. Hydrogen bond is defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (F, O, N) of the same or another molecule.
2. Intermolecular hydrogen bond and Intramolecular hydrogen bond.
3. Compounds containing hydrogen bonds show higher melting and boiling points. Compounds whose molecules can form hydrogen bonds with water molecules are soluble in water.
Question 2.
Classify the following compounds according to their
shape.
BeF2, BeCl2, CH4, BF3, PCl5, SF6, SbCl5 NH4+, SiF4, AlCl3.
Answer:
Linear – BeF2, BeCl2
Trigonal planar-AlCl3, BF3
Tetrahedral – CH4, NH4+, SiF4
T rigonal bipyramidal – PCl5, SbCl5
Question 3.
Benzene is an example of a compound exhibiting resonance.
1. What is meant by resonance?
2. Explain the resonance of ozone.
Answer:
1. When a molecule cannot be represented by a single structure but its characteristic properties can be described by two or more different structures, then the actual molecule is said to be a resonance hybrid of these canonical structures.
2. The resonance in ozone can be represented by the following structures:
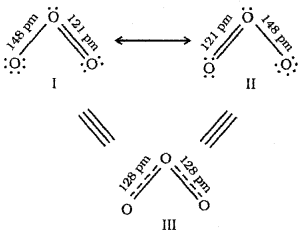
According to structures I and II, there is one single bond and one double bond in ozone molecule. But experiments show that both the oxygen- oxygen bonds are equal and the bond length 128 pm) is intermediate between single (148 pm) and double bond (121 pm) lengths. Hence it is assumed that ozone is a resonance hybrid (structure III) of structures I and II.
Question 4.
Match the following:
| No. of electrons pairs | Shape of molecule | Examples |
| 2 | Trigonal planar | SF6 |
| 4 | Linear | BeF2, BeCl2 |
| 3 | Tetrahedral | BF3, AlCl3 |
| 6 | Octahedral | CH4, SiF4 |
Answer:
| No. of electrons pairs | Shape of molecule | Examples |
| 2 | Linear | BeF2, BeCl2 |
| 4 | Tetrahedral | CH4, SiF4 |
| 3 | Trigonal planar | BF3, AlCl3 |
| 6 | Octahedral | SF6 |
Question 5.
In the formation of methane, carbon undergoes sp³ hybridisation.
- What do you mean by sp³ hybridisation?
- Give the % s-character and p-character of an sp³ hybrid orbital.
- What is the bond angle in methane?
- What is the geometry of methane molecule?
Answer:
- sp³ hybridisation involves mixing up of one – s and three-p orbitals of the valence shell of an atom to form four sp³ hybrid orbitals of equivalent energies and shape.
- Each sp³ hybrid orbital has 25% s-character and 75% p-character.
- The angle between the sp3 hybrid orbitals in methane is 109°28′.
- Tetrahedron.
Question 6.
Complete the following table:
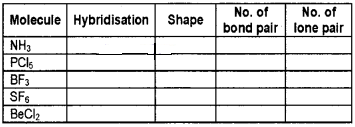
Answer:
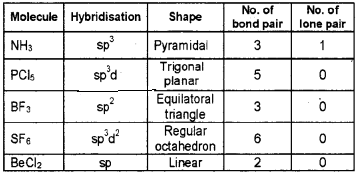
Question 7.
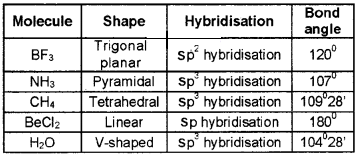
Fill in the blanks:
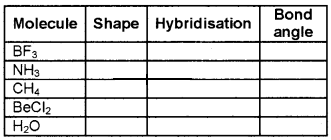
Answer:
Question 8.
Dipole moment is used to predict the shape of
molecules.
1. Justify the statement based on the shapes of CO2 and H2O.
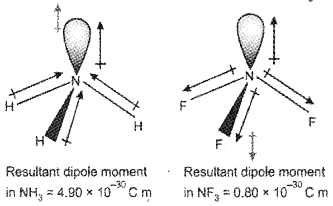
2. Which is having a high dipole moment? NH3 or NF3? Why?
Answer:
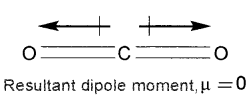
1. Carbon dioxide is a linear molecule in which the two C=0 bonds are oriented in opposite directions at an angle of 180°. Hence the two C=0 bond dipoles cancel each other and the resultant dipole moment of CO2 is zero. Thus CO2 is a non-polar molecule
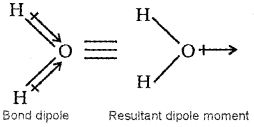
On the other hand, water molecule has a bent structure in which two O-H bonds are oriented at an angle of 104.5°. Therefore, the bond dipoles of two O-H bonds do not cancel each other and the molecule will have a net dipole moment (1.85D).
2. The dipole moment of NH3 is higher than that of NF3. In both cases, the central N atom has a lone pair whose orbital dipole points away from the N atom. In NH3 the orbital dipole due to the lone pair is in the same direction as the resultant bond dipole of the three N-H bonds. On the other hand, in the case of NF3, the resultant dipole of the three N-F bonds is in the opposite direction to the orbital dipole due to the lone pair. Thus, the orbital dipole due to the lone pair decreases the effect of the resultant N-F bond moments, which results in the low dipole moment of NF3.
Question 9.
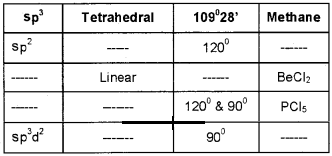
The geometry of a covalent molecule is related to the hybridisation involved in the central atom. Complete the following table:
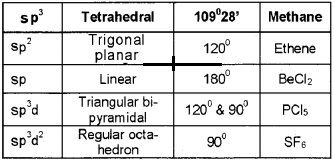
Answer:
Question 10
Depending upon the type of overlapping, covalent bonds are of two types.
a) Name them and give any two differences between them.
b) Find the total number of these two types of bonds in propane and 2-butene.
Answer:
a) Sigma (σ) bond and pi (π) bond.
Sigma (σ) bond:
This type of covalent bond is formed by the end to end overlapping of half-filled atomic orbitals along the internuclear axis. The overlap is also known as head on overlap or axial overlap. The electrons constituting sigma bond are called sigma electrons.
Pi (π) bond:
This type of covalent bond is formed by the lateral or sidewise overlap of half-filled atomic orbitals. The atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the internuclear axis.
b) 10 σ bond in propane
11 σ bond and 1π bond in 2-butene
Plus One Chemistry Chemical Bonding and Molecular Structure NCERT Questions and Answers
Question 1.
Explain the formation of a chemical bond. (2)
Answer:
According to the Kossel-Lewis approach, the formation of a chemical bond between the two atoms takes place either by the transference of electrons or by mutual sharing of electrons. However, according to the modem view the formation of chemical bond between the two approaching atoms occurs only if there is a net decrease of energy because of attractive and repulsive forces.
Question 2.
Write the favourable conditions for the formation of ionic bond. (2)
Answer:
Ionic bond is formed by transference of electrons from one atom to another. The favourable conditions for its formation are:
- Low ionisation enthalpy of element forming cation.
- More negative value of electron gain enthalpy of element forming the anion and
- High value of lattice enthalpy of the compound formed.
Question 3.
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that in ammonia. Discuss. (2)
Answer:
The difference in bond angles is due to the different numbers of lone pairs and bond pairs in the two species. In NH3, the N atom has two lone pairs and three bond pairs while in H2O, the O atom has two lone pairs and two bond pairs. The repulsive interactions of lone pairs and bond pairs in water are relatively more than those in NH3. Hence, bond angle around central atom in water is relatively smaller (104.5°) than that in NH3 molecule (107°).
Question 4.
Is there any change in the hybridisation of B and N atoms as a result of the following reaction? (2)
BF3 + NH3 → F3B.NH3
Answer:
During combination of species BH3 and NH3, N atom of NH3 is donor and B atom of BF3 is acceptor. The hybrid state of B in BF3 is sp² and that of N in NH3 is sp³. In the compound F3B+-NH3– both N and B atoms are surrounded by four bond pairs. Thus, the hyrid state of both is sp³. Hence during the reaction the hybrid state of B changes from sp² to sp³ but that of N remains the same.
Question 5.
Define hydrogen bond. Is it weaker or stronger than the van der Waals’ forces? (2)
Answer:
Hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (F, O or N) of another molecule. Hydrogen bond is stronger than van der Waals’forces because it is a strong dipole-dipole interaction. The van der Waals’ forces, on the other hand, are weak dispersion forces.
We hope the Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 4 Chemical Bonding and Molecular Structure help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 4 Chemical Bonding and Molecular Structure, drop a comment below and we will get back to you at the earliest.
