Plus One Chemistry Chapter Wise Questions and Answers Chapter 3 Classification of Elements and Periodicity in Properties is part of Kerala Plus One Chemistry Chapter Wise Questions and Answers. Here we have given Plus One Chemistry Chapter Wise Questions and Answers Chapter 3 Classification of Elements and Periodicity in Properties.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 3 Classification of Elements and Periodicity in Properties
Plus One Chemistry Classification of Elements and Periodicity in Properties One Mark Questions and Answers
Question 1.
Which of the following is not a Dobereiner triad?
a) Cl, Br, I
b) Ca, Sr, Ba
c) Li, Na, K
d) Fe, Co, Ni
Answer:
d) Fe, Co, Ni
Question 2.
The elements of s-block and p-block are collectively called ___________
Answer:
Representative elements
Question 3.
The cause of periodicity of properties is
a) Increasing atomic radius
b) Increasing atomic weights
c) Number of electrons in the valence shell
d) The recurrence of the similar outer electronic configuration
Answer:
d) The recurrence of the similar outer electronic configuration
Question 4.
Halogen with highest ionization enthalpy is ___________ .
Answer:
Fluorine
Question 5.
Which of the following represents the most electropositive element?
a) [He]2s1
b) [He]2s2
c) [Xe]6s1
d) [Xe]6s2
Answer:
c) [Xe]6s1
Question 6.
Second electron gain enthalpy is
Answer:
always positive
Question 7.
Correct order of polarising power is
a) Cs+ < K+ < Mg2+ < Al3+
b) Al3+ < Mg2 + K+ < Cs+
c) Mg2+ < Al3+ < K+ < Cs+
d) K+ < Cs+ < Mg2+ < Al3+
Answer:
a) Cs+ < K+ < Mg2+ < Al3+
Question 8.
The IUPAC name of the element with atomic number is 109 is ___________
Answer:
Une
Question 9.
The size of isoelectronic species F~, Ne and Na+ is affected by
a) Nuclear charge
b) Principal quantum number.
c) Electron-electron interaction in outer orbitals.
d) None of the factors because their size is the same.
Answer:
Nuclear charge as nuclear charge is high the size is small.
Question 10.
In transition elements the differentiating electron occupies (n-1)d sublevel in preference to ______________
Answer:
np level
Plus One Chemistry Classification of Elements and Periodicity in Properties Two Mark Questions and Answers
Question 1.
The arguments made by two students are as given:
Student 1: ‘Hydrogen belongs to Group 1.’
Student 2: ‘Hydrogen belongs to Group 17.’
1. Who is right?
2. What is your opinion?
Answer:
1. Nobody is right.
2. Hydrogen has a one s-electron and hence can be placed in group 1 (alkali metals). It can also • gain an electron to achieve a noble gas arrangement and hence it can behave similar to a group 17 (halogen family) element. Because it a special case, hydrogen is placed separately at the top of the Periodic Table.
Question 2.
Match the following:
| Sodium | f-block |
| Oxygen | s-block |
| Uranium | d-block |
| Silver | p-block |
Answer:
Sodium – s-block
Oxygen – p-block
Uranium – f-block
Silver – d-block
Question 3.
- Which one has greater size, Na or K?
- Justify your answer.
Answer:
1. K
2. K comes below Na in the Periodic Table. The atomic size increases down the group due to the fact that the inner energy levels are filled with electrons, which serve to shield the outer electrons from the pull of the nucleus.
Question 4.
The general characteristics of a particular block of elements is as given:
They are highly electropositive, soft metals. They are good reducing agents. They lose the outermost electron(s) readily to form 1+ ion of 2+ ion.
- Which block has these general characteristics?
- Write down two general characteristics of p-block.
Answer:
1. s-block
2. The p-block contains metals, non-metals and metalloids. They form ionic as well as covalent compounds.
Question 5.
The variation in electron gain enthalpies of elements is less systematic than for ionization enthalpies. Out of oxygen and sulphur which has greater negative value for electron gain enthalpy? Justify.
Answer:
Sulphur has greater negative value (-200 kJ mol1) for electron gain enthalpy compared to that of oxygen (-141 kJ mol1). This is because, due to smaller size of oxygen the added electron experiences much repulsion from the electrons present in the shell. Due to large size, the electron-electron repulsion is much less in sulphur.
Question 6.
Some elements are given. Li, Cs, Be, C, N, O F, I, Ne, Xe.
- Arrange the above elements in the increasing order of ionization enthalpy.
- Arrange the given elements in the decreasing order of negative electron gain enthalpy.
Answer:
- Cs < I < Li < Xe < Be < C < N < O < F < Ne
- Cl > F > O > N > C > Be > Li > I >C s > Xe > Ne
Plus One Chemistry Classification of Elements and Periodicity in Properties Three Mark Questions and Answers
Question 1.
Analyse the given figure and answer the questions that follow.
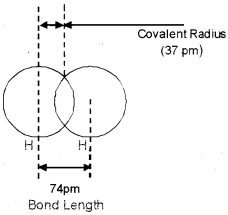
- What is meant by atomic radius?
- Explain covalent radius.
- Write down another two types of terms expressed as atomic size.
Answer:
- Atomic radius is defined as the distance from the centre of the nucleus of the atom to the outermost shell of electrons.
- Covalent radius is defined as one half of the distance between the centre of nuclei of two similar atoms bonded by a single covalent bond.
- Vander Waals’ radius, Metallic radius
Question 2.
Consider the statement: The element with 1s2
configuration belongs to the p-block.’
- Identify the element.
- Do you agree with this statement?
- Justify.
Answer:
- Helium
- Yes
- Strictly, helium belongs to the s-block but its positioning in the p-block along with another group 18 elements is justified because it has a completely filled valence shell (1s²) and as a result, exhibits characteristic of other noble gases.
Question 3.
The properties of elements are a periodic function of
their atomic weights.
- Who proposed this law?
- Can you see anything wrong in this law? If yes, justify your answer.
- State modem periodic law.
Answer:
- Mendeleev
- Yes, atomic number is the more fundamental property of an element than atomic mass.
- The physical and chemical properties of the elements are periodic functions of their atomic numbers.
Question 4.
1. Define ionisation enthalpy.
2. IE1 <IE2 <IE3
What is meant by this? Justify.
Answer:
1. Ionisation enthalpy is the amount of energy required to remove the most loosely bound electron from an isolated gaseous atom in its ground state.
2. It shows the increasing order the magnitude of successive ionization enthalpies. As the positive charge of the ion increases, it becomes more difficult to remove the valence electron due to increase in effective nuclear charge.
Question 5.
Say whether the following are true or false:
- On moving across a period ionization enthalpy decreases.
- Mg is bigger than Cl.
- Ionization enthalpy of Li is less than that of K.
Answer:
- False
- True
- False
Question 6.
Analyze the graph given below:
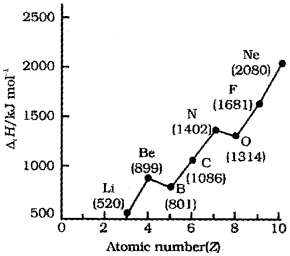
1. Identify the graph.
2. Account for the following observations:
i) ‘Ne’ has the maximum value of ∆iH.
ii) In the graph from Be to B, ∆iH decrease.
Answer:
1. Graph showing the variation of ionisation enthalpy (∆iH) with atomic number (Z) of the elements of second period.
2. i) ‘Ne’ is an inert gas and it has closed electron shell with stable octet electronic configuration. Hence it has the maximum ionisation enthalpy in the second period.
ii) Be has completely filled electronic configuration and a more stable. So ionization enthalpy is high
Question 7.
Study the graph and answer the questions that follow:
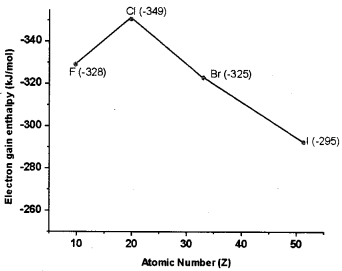
1. On moving down a group what happens to electron gain enthalpy?
2. Why chlorine shows more negative electron gain enthalpy than fluorine? ,
Answer:
1. On moving down a group, electron gain enthalpy becomes less negative because the size of the atom increases and the added electron would be farther from the nucleus.
2. Due to small size of F, the added electron goes to the smaller 2p quantum level and suffers significant repulsion from the other electrons present in this level. But due to big size of Cl, the added electron goes to the n = 3p quantum level and occupies a larger region of space and the electron-electron repulsion is much less.
Question 8.
Electron gain enthalpy is an important periodic property.
1. What is meant by electron gain enthalpy?
2. What are the factors affecting electron gain enthalpy?
3. How electron gain enthalpy varies on moving across a period? Justify.
Answer:
1. Electron gain enthalpy(∆egH) is the enthalpy change accompanying the process of addition of an electron to a neutral gaseous atom to convert it into a negative ion.
2. Effective nuclear charge, atomic size, electronic configuration
3. Electron gain enthalpy becomes more negative with increase in the atomic number across a period. The effective nuclear charge increases from left to right across a period and consequently, it will be easier to add an electron to a smaller atom since the added electron on average would be closer to the nucleus. Thus more energy is released.
Question 9.
1. The electron gain enthalpies of Be and Mg are positive. What is your opinion? Justify.
2. Electron gain enthalpies of nobles gases have large positive values. Why?
Answer:
1. The statement is correct. Electron gain enthalpies of Be (+240 kJ/mol) and Mg (+230 kJ/mol) are positive because they have stable electronic configurations with fully filled s-orbitals (Be – 2s², Mg – 3s²). Hence, the gain of electron is highly endothermic.
2. Noble gases have stable octet electronic configuration of ns2 np6 (except He -1 s²) and they have practically no tendency to accept additional electron. They have large positive electron gain enthalpies because the electron has to enter the next higher principal quantum level leading to a very unstable electronic configuration. Hence, energy has to be supplied to add an extra electron.
Question 10.
During a group discussion, a student argued that ionization enthalpy depends only upon electronic configuration.
1. Do you agree? Comment.
2. Define shielding effect/screening effect.
3. Is there any relation between ionization enthalpy and shielding effect/screening effect? Explain.
Answer:
1. No. In addition to electornic configuration, ionization enthalpy depends upon other factors like atomic size, nuclear charge and shielding effect/screening effect.
2. In multi-electron atoms, the nuclear charge experienced by the valence electron will be less than the actual charge on the nucleus because it is shielded by inner core of electrons. This is called shielding effect or screening effect.
3. Yes. Ionization enthalpy decreases with increase in shielding effect/screening effect of inner electrons. This is because as a result of shielding of the valence electron by the intervening core of electrons it experiences a net positive charge which is less than the actual charge of the nucleus. In general, shielding is effective when the orbitals in the inner shells are completely filled.
Question 11.
a) Which of the following has higher first ionization enthalpy, N or O? Justify.
b) Which one is bigger, For F ? Why?
Answer:
1. N. This is because in N, three 2p-electrons reside in different atomic orbitals in accordance with Hund’s rule () whereas in O, two the four 2p-electrons must occupy the same 2p-orbital resulting in an increased electron-electron repulsion (). Consequently, it is easier to remove the fourth 2p-electron from O than it is, to remove one of the three 2p-electrons from N.
2. F (136 pm) is bigger than F (72 pm). An anion is bigger than its parent atom. Addition of one electron in F results in increased repulsion among the electrons and a decrease in effective nuclear charge. Thus, attraction between nucleus and the electrons decreases and hence size increases.
Question 12.
Electronegativity differs from electron gain enthalpy.
- Do you agree?
- What do you mean by electronegativity?
Answer:
- Yes.
- Electronegativity is defined as the tendency of an atom in a chemical compound to attract the shared pair of electrons to itself.
Question 13.
Ionization enthalpy is an important periodic property.
1. What is the unit in which it is expressed?
2. What are the factors influencing ionization enthalpy?
3. How ionisation enthalpy varies in the periodic table?
Answer:
1. kJ mol-1.
2. Atomic/ionic radius, nuclear charge, shielding effect/screening effect, penetration effect and electronic configuration.
3. Ionization enthalpy generally increases with increase in atomic number across a period due to regular increase in nuclear charge and decrease in atomic size. Thus, the attractive force between the nucleus and the electron cloud increases. Consequently, the electrons are more and more tightly bound to the nucleus.
Ionisation enthalpy generally decreases from top to bottom a group: This is because the effect of increased nuclear charge is cancelled by the increase in atomic size and the shielding effect. Consequently, the nucelar hold on the valence electron decreases gradually and ionization enthalpy decreases.
Question 14.
The second period elements show anomalous ‘ behaviour.
1. Give reason.
2. What are the anomalous properties of second period elements?
Answer:
1. The first element is each group belong to the second period. The difference in behaviour of these elements from the other elements of the same group can be attributed to the following factors:
- Small atomic size
- Large charge/radius ratio
- High electronegativity
- Absence of d-orbtials in the valence shell
2. The important anomalous properties of second period elements are: diagonal relationship, maximum covalence of four and ability to form pπ —pπ multiple bonds.
Question 15.
Atomic size, valency, ionization enthalpy, electron gain enthalpy and electronegativity are the important periodic properties of elements.
1. What do you mean by periodicity?
2. Periodic properties are directly related to ___________
Answer:
1. The periodical repetition of elements with similar properties after certain regular intervals when the elements are arranged in the order of increasing atomic numbers is called periodicity,
2. Electronic configuration.
Question 16.
1. Which is the element among alkali metals having lowest ionization enthalpy?
2. What is meant by valence of an element? How it varies in the periodic table?
3.
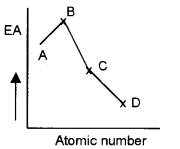
Identify the elements A, B, C and D, if the graph represents halogens.
Answer:
1. Fr
2. Valence of an element is the combining capacity of that element. In the case of representative elements the number of valence electrons increases from 1 to 8 on moving across a period, the valence to the element with respect to H and Cl increases from 1 to 4 and then decreases from 4 to zero. On moving down a group, the number of valence electrons remains same and, therefore, all the elements in a group exhibit same valence.
3. A = F, B = Cl, C = Br, D = I
Question 17.
Li and Mg belonging to first and second group in periodic table respectively resemble each other in many respects.
1. Name the relationship.
2. B can only form [BF4]– ion while Al can form [AIF6]3-, though both B and Al belong to group 13. Justify.
Answer:
1. Diagonal relationship.
2. This is because B, being a second period element has a maximum covalence of 4. It cannot expand its covalence beyond 4 due to absence of d-orbitals. But Al, being a third period element has vacant d-orbitals in its valence shell and hence can expand its covalence beyond 4.
Question 18.
During a group discussion a student argues that both oxidation state and valence are the same.
1. Do you agree?
2. Justify taking the case of [AICI(H2O)5]2+.
Answer:
1. No. Valence refers to the combining capacity of an element whereas oxidation state is the charge assigned to an element in a compound based on the assumption that the shared electron in a covalent bond belongs entirly to the more electronegative element.
2. In [AICI(H2O)5]2+the valence of Al is 6 while its oxidation state is +3.
Question 19.
Among the elements of the third period, identify the element
- With highest first ionization enthalpy.
- That is the most reactive metal.
- With the largest atomic radius.
Answer:
- Ar
- Na
- Na
Question 20.
A cation is smaller than the corresponding neutral atom while an anion is larger. Justify.
Answer:
A cation is smaller than its parent atom because it has fewer number of electrons while its nuclear charge remains the same. The size of an anion will be larger than that of the parent atom because the addition of one or more electrons would result in increased . repulsion among the electrons and a decrease in effective nuclear charge.
Question 21.
1. How the metallic character varies in the periodic table?
2. Categorize the following oxides into acidic, basic, neutral and amphoteric:
Al2O3, Na2O, CO2, Cl2O7, MgO, CO, As2O3, N2O
Answer:
1. The metallic character decreases from left to right across the period due to increase in ionization enthalpy along a period which makes loss of electrons difficult. From top to bottom a group metallic character increases due to decrease in ionization enthalpy. Thus, metallic character decreases diagonally from left bottom to right top of the periodic table.
2. Acidic oxides: CO2, Cl2O7
Basic oxides: Na2O, MgO
Neutral oxides: CO, N2O
Amphoteric oxides: Al2O3, As2O3
Question 22.
A group of ions are given below:
Na+, Al3+, O2-, Ca2+, Mg2+, F–, N3-, Br–
1. Find the pair which is not isoelectronic.
2. Arrange the above ions in the increasing order of size.
Answer:
1. Ca2+ and Br
2. Al3+ < Mg2+ < Na+ < F– < O2- < N3- < Ca2+ < Br–
Plus One Chemistry Classification of Elements and Periodicity in Properties Four Mark Questions and Answers
Question 1.
Statement 1: ‘Atomic mass is the fundamental property of an element.’
Statement 2: ‘Atomic number is a more fundamental property of an element than its atomic mass.’
1. Which statement is correct? Justify your answer.
2. Name the scientist who proposed this statement? What observation led him to this conclusion?
Answer:
1. Statement 2. Atomic number indicates the number of electrons present in an element. Most of the chemical properties of an element depend on its electronic configuration,
2. Henry Moseley. He observed regularities in the characteristic X-ray spectra of the elements. A plot of √υ (where u is the frequency of the X-rays emitted) against atomic number (Z) gave a straight line and not the plot of √υ vs atomic mass.
Question 2.
Atoms possessing stable configuration have less tendency to loss electrons and consequently will have high value of ionization enthalpy.
1. Justify this statement by taking the case of half-filled and completely filled electronic configurations.
2. The noble gases have highest ionization enthalpies in each respective periods. Why?
Answer:
1. Atoms with half-filled and completely filled electronic configurations have extra stability due to symmetric distribution of electrons and maximum exchange energy. Hence, more energy is required for the removal of their electrons. Elements like N (1s² 2s² ), P (1s² 2s² 2p6 3s² ) etc. possessing half-filled shells have high ionization enthalpies.
Elements like Be ((1s² 2s²), Mg(1s² 2s² 2p6 3s²) etc. having completely filled shells show high values of ionization enthalpy.
2. Noble gases have closed electron shells and very stable octet electronic configurations (except He). Hence, maximum amount of energy is required to remove their valence electron.
Question 3.
Mendeleev arranged the elements in the order of increasing atomic weights.
a) Write down the merits of Mendeleev’s periodic table.
b) What are the demerits of Mendeleev’s periodic table?
Answer:
a) Merits of Mendeleev’s periodic table:
- Study of elements – Elements are classified into groups with similar properties, thus facilitating the study of properties of elements.
- Prediction of new elements – Mendeleev left certain vacant places in his table which provided a clue for the discovery of new elements, e.g. Eka-AI (for Ga), Eka-Si (for Ge).
- Determination of correct atomic weights – With the help of this table, doubtful atomic weights of some elements were corrected.
b) Demerits of Mendeleev’s periodic table:
- Position of hydrogen was not certain.
- Anomalous pairs of elements – Certain elements of higher atomic weight preceed those with lower atomic weight, e.g. I (at.wt. 127) was placed after Te (at.wt. 128).
- Lanthanides and Actinides are not given proper places in this periodic table.
- No proper position for isotopes.
Question 4.
1. Name any three numerical scales of electronegativity.
2. How electronegativity varies in the periodic table? Justify.
Answer:
1. Pauling scale, Mullimen-Jaffe scale, Allred- Rochow scale.
2. Electronegativity generally increases from left to right a period and decreases from top to bottom in a group. This is because, from left to right across a period atomic size decreases and attraction between the valence electrons and the nucleus increases. From top to bottom in a group atomic size increases and attraction between the valence electrons and the nucleus decreases.
Question 5.
1. First ionization enthalpy of Na is lower than that of Mg. But its second ionization enthalpy is higher than that of Mg. Explain.
2. Which one is smaller, Na or Na+? Give reason.
Answer:
1. By the removal of one electron from Na it gets
the stable octet configuration of Ne. But when the first electron is removed from Mg it gets the unstable configuration of Na. It requires more energy due to small size and greater nuclear charge of Mg. In the case of Na the second electron is to be removed from a stable octet configuration which requires more energy than the removal of second electron from Mg.
2. Na+ (95 pm) is smaller than Na (186 pm). Acation is smaller than its parent atom. Na+ has fewer number of electrons (10 electrons) compared to Na (11 electrons). But the nuclear charge remains the same in both. Thus, effective nuclear charge per electron is greater in Na+. Thus, the attraction between nucleus and the remaining electrons increases and size decreases.
Question 6.
Removal of electron becomes easier on moving down the group.
1. Comment the above statement based on ionization enthalpy.
2. How electronic configuration influences the ionization enthalpy value?
Answer:
1. On moving from top to bottom in a given group, size of the atom increases and ionisation enthalpy decreases. Hence, it becomes easier to remove the valence electron.
2. Atoms with octet configuration, half-filled and completely filled configurations have extra stability and hence have higher values of ionization enthalpy.
Question 7.
The energy released during the addition of an electron to an isolated neutral atom is called electron gain enthalpy.
1. Explain how electron gain enthalpy differ from electronegativity.
2. The second ionisation enthalpy of an element is always greater than the first ionisation enthalpy. Give reason.
Answer:
1. Electron gain enthalpy(AegH) is the enthalpy change accompanying the process of addition of an electron to a neutral gaseous atom to convert it into a negative ion. It is a quantitative property of an isolated gaseous atom, which can be measured. Whereas, electronegativity is a qualitative measure of the ability of an atom in a chemical compound to attract shared electrons to itself. It is not a measureable quantity.
2. This is because to remove second electron from a positively charged ion more amount of energy is required due to increase in effective nuclear charge.
Question 8.
The physical and chemical properties of elements are periodic functions of their atomic numbers.
1. The atomic number of an element ‘X’ is 19. Write the group number, period and block to which X’ belong in the periodic table.
2. Name the element with
i) highest electronegativity and
ii) highest electron gain enthalpy
Answer:
1. The element is K.
19K= 1s² 2s² 2p6 3s² 3p6 4s1
Group number = 1
Period number = 4
Block = s-block
2. i) Fluorine
ii) Chlorine
Plus One Chemistry Classification of Elements and Periodicity in Properties NCERT Questions and Answers
Question 1.
What is the basic theme of organisation in the periodic table? (2)
Answer:
The basic theme of organisation of elements in the periodic table is to facilitate the study of the properties of all the elements and their compounds. On the basis of similarities in chemical properties, the various elements have been divided into different groups. This has made the study of elements simple because their properties are now studied in the form of groups rather than individually.
Question 2.
What is the basic difference in approach between Mendeleev’s Periodic Law and the Modern Periodic Law? (2)
Answer:
Mendeleev Periodic Law states that the properties of the elements are a periodic function of their atomic weights whereas Modern Periodic Law states that the properties of elements are a periodic function of their atomic numbers. Thus, the basic difference in approach between Mendeleev’s Periodic Law and Modern Periodic Law is the change in basis of arrangements of elements from atomic weight to atomic number.
Question 3.
Consider the following species. N3-, O2-, F–, N2+, Mg2+ and Al3+ (2)
1. What is common in them?
2. Arrange them in order of increasing ionic radii.
Answer:
1. Each one of these ions contains 10 electrons and
hence these are isoelectronic ions,
2. The ionic radii of isoelectronic ions decrease with the increase in the magnitude of the nuclear charge. Among the isoelectronic ions: N3-, O2-, F–, Na+, Mg2+ and Al3+, nuclear charge increase in the order:
N3-< O2- < F– < Na+ < Mg2+ < Al3+
Therefore, the ionic radii decrease in the order:
N3- > O2- > F– > Na+ > Mg2+ > Al3+
Question 4.
Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
(a) Valence principal quantum number (n)
(b) Nuclear charge (Z)
(c) Nuclear mass
(d) Number of core electrons (1)
Answer:
Nuclear mass does not affect the valence shell. Thus, option (c) is the correct answer.
Question 5.
Considering the elements F, Cl, O and N, the correct order of their chemical reactivity in terms of oxidising property is: (1)
a) F > Cl > O > N
b) F > O > Cl > N
c) Cl > F > O > N
d)0>F>N>CI
Answer:
a) F > Cl > O > N
Across a period, the oxidising character increases from left to right. Therefore, among F, O and N, oxidising power decreases in the order: F > O > N. However, within a group, oxidising power decreases from top to bottom. Thus, F is a stronger oxidising agent than Cl. Thus, overall decreasing order of oxidising power is F > Cl > O > N and the choice (a) is correct.
We hope the Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 3 Classification of Elements and Periodicity in Properties help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 3 Classification of Elements and Periodicity in Properties, drop a comment below and we will get back to you at the earliest.
