Plus One Chemistry Chapter Wise Questions and Answers Chapter 11 The p-Block Elements is part of Kerala Plus One Chemistry Chapter Wise Questions and Answers. Here we have given Plus One Chemistry Chapter Wise Questions and Answers Chapter 11 The p-Block Elements.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 11 The p-Block Elements
Plus One Chemistry The p Block Elements One Mark Questions and Answers
Question 1.
The aqueous solution of borax is
a) Acidic
b) Alkaline
c) Neutral
d) Amphoteric
Answer:
b) Alkaline
Question 2.
Say TRUE or FALSE.
Boron in aqueous solution forms B3+ ion.
Answer:
False
Question 3.
Which of the halide of group 14 does not exist?
a) CF4
b) Cl4
c) SiF4
d) Pbl4
Answer:
d) Pbl4
Question 4.
Orthoboric acid, H3BO3 is a
a) Protonic acid
b) Arrhenius acid
c) Lewis acid
d) Bronsted-Lowery acid
Answer:
c) Lewis acid
Question 5.
The zeolite used as a catalyst in petrochemical industries for cracking of hydrocarbons and isomerization is _________ .
Answer:
ZSM-5
Question 6.
Dry ice is __________ .
Answer:
Solid CO2
Question 7.
Thermodynamically most stable allotrope of carbon is __________ .
Answer:
Graphite
Question 8.
The alkali metal used in solar cells is __________ .
Answer:
Cs (Caesium)
Question 9.
Acidity is in the order
Answer:
BBr3 > BCl3 > BF3
Question 10.
AlCl3 fumes in moist air because
Answer:
HCl is formed due to hydrolysis in moist air
Plus One Chemistry The p Block Elements Two Mark Questions and Answers
Question 1.
Silicon belongs to the carbon family. Graphite is an important allotrope of carbon. But Si does not form an analogue of graphite. What are the possible reasons?
Answer:
Silicon atom is much bigger in size than carbon. Si-Si bond energy is less than C-C bond energy and also Si does not form compounds in sp² hybrid state.
Question 2.
Boron is an element with atomic number 5.
a) Write down the electronic configuration of boron.
b) Mention any two uses of boron.
c) Write down some compounds of boron.
Answer:
a) 1s²2s²2p¹
b) To increase the hardness of the steel.
Used as the semiconductor in electronic devices.
c) Diborane, borax, orthoboric acid, boron trifluoride
Question 3.
Answer the questions, with the help of the following:
- Hard solid
- Melting point above 450 K.
- Four allotropic forms are known.
- Low electrical conductivity.
- Mass number! 3
1. Which is the element?
2. Write any two uses of this element.
3. ![]()
4. Write any three compounds of this element.
Answer:
1. Boron
2. To increase hardness of steel
As semiconductor in electric devices
3. 2B
4. Diborane, Borax, Boric acid
Question 4.
1. Write any two allotropic forms of carbon.
2. C + ½ O2 → …………….
3. Write any two uses of carbon monoxide.
4. How is carbon dioxide produced?
5. What is the hardest element/form of an element in the world?
Answer:
1. Diamond, Graphite
2. CO (Carbon monoxide)
3. Reducing agent in metallurgy
Used in the manufacture of methanol
4. Carbon dioxide is formed by the complete combustion of carbon.
C + O2 → CO2
5. Diamond
Question 5.
From the compounds of group 14 elements write an appropriate example for each of the following:
| No. | Type of compound | Name/ Formulae of example |
| 1. | A strong reducing oxide | |
| 2. | A giant covalent oxide | |
| 3. | A strongly reducing chloride | |
| 4. | A covalent chloride not hydrolysed by water |
Answer:
1-CO
2-SiO2
3-SnCl2
4-CCl4
Question 6.
Diborane has an unusual structure. Justify the statement with figure.
Answer:
In diborane, each Batom uses sp³ hybrid orbitals for bonding. Out of the four sp³ hybrid orbitals on each B atom, one is without an electron. The terminal B-H bonds are normal 2-center-2-electron bonds but the two bridge bonds are 3-center-2-electron bonds. The 3-centre-2-electron bridge bonds are also referred to as banana bonds.
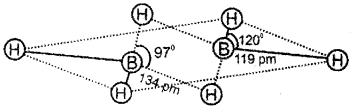
Question 7.
Match the following:
| A | B |
| CO | i. A semi conductor in electronic devices |
| H2 | ii. Reducing agent in metallurgy |
| O3 | iii. Thermal decomposition of Ammonia |
| Boron | iv. Used as a chemical reagent in organic chemistry |
Answer:
CO – ii,
N2 – iii,
O3 – iv,
Boron – i
Question 8.
BCl3 fumes in moist air.
1. Give a reason.
2. Write down a balanced equation that can reveal the answer.
Answer:
1. When the water of moist air reacts with boron halide hydrogen chloride is formed which causes fumes,
2. BCl3 + 3H2O → H3BO3 + 3HCl
Question 9.
‘Generally, non-metal oxides are basic.’
1. Do you agree?
2. What do you mean by oxides?
3. Which are the different types of oxides?
4. Give examples for each type of oxides.
Answer:
1. No. Generally, non-metallic oxides are acids.
2. The binary compounds formed by the combination of oxygen with metals or nonmetals are called oxides.
3. Acidic oxides, basic oxide, amphoteric oxide, neutral oxide.
4. Acidic oxide → SO2, NO2
Basic oxide → MgO
Amphoteric oxide → Al2O3, ZnO
Neutral oxide → CO, N2O
Question 10.
Match the following:
1. Borane – H3BO3
2. Boric acid – Na2B4O7.10H2O
3. Borax – Amphoteric oxide
4. Al2O3 – Boron hydride
Answer:
1. Borane – Boron hydride
2. Boric acid – H3BO3
3. Borax – Na2B4O7.10H20
4. Al2O3 – Amphoteric oxide
Question 11.
How diborane reacts with
1. Oxygen?
2. Water?
Answer:
1. Diborane catches fire spontaneously upon exposure to air. It burns in oxygen releasing an enormous amount of energy.
B2H6 + 3O2 → B2O2 + 3H2O; ∆rHΘ -1976 kJ mol-1
2. Diborane is readily hydrolysed by water to give boric acid.
B2H6(g) + 6H2O(l) → 2B(OH)3(aq) + 6H2(g)
Question 12.
1. CCl4 cannot be hydrolysed. Give reason.
2. Draw the structure of the dimer of AlCl3.
Answer:
1. Carbon has no d orbitals to accommodate the lone pair of electrons from oxygen atom of H20.
2.
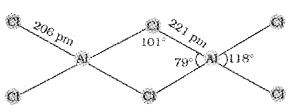
Question 13.
1. Draw the structure of boric acid.
2. Starting from borax how will you prepare boric acid? (Write the chemical equation).
Answer:
1.
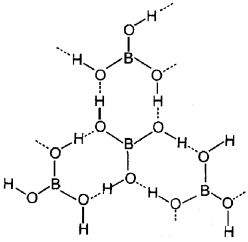
2. Na2B4O7 + 2HCl + 5H2O → 2NaCl + 4B(OH)3.
Question 14.
CO2 is a gas but SiO2 is solid. Give reason.
Answer:
CO2 exists as discrete molecules due to the formation of pπ -pπ double bond between carbon and oxygen. But SiO2 has a three-dimensional network structure in which each Si atom is covalently bonded in a tetrahedral manner to four oxygen atoms.
Question 15.
1. What are zeolites?
2. What is ZSM-5?
Answer:
1. Zeolites are aluminosilicates with a three-dimensional network structure in which Al atoms replace few Si atoms. Cations such as Na+, K+ or Ca2+ balance the negative charge of aluminosilicate anion.
2. ZSM-5 is a zeolite catalyst used in the petrochemical industry to convert alcohol directly into gasoline.
Plus One Chemistry The p Block Elements Three Mark Questions and Answers
Question 1.
Explain the following:
1. Allotropy
2. Coke and Charcoal
Answer:
1. The phenomenon of existence of an element in two or more forms, which have different physical properties but almost similar chemical properties, is called allotropy and the different forms are called allotropes.
2. Coke and Charcoal are amorphous forms of carbon. Coke is formed-by the destructive distillation of coal. It is used as a fuel and also as a reducing agent in metallurgy.
Question 2.
Give justifications.
1. The first ionisation enthalpy of carbon is greater than that of boron, whereas the reverse is correct for the second ionisation enthalpy.
2. Graphite is a better lubricant on moon than that on earth.
Answer:
1. This is because carbon has greater nuclear charge. For second ionisation enthalpy, an electron is to be removed from 2p of carbon while in boron the second electron is in 2s orbital. Removal of a 2s electron is more difficult due to the high penetrating power of 2s orbital.
2. Graphite contains hexagonal sheets of carbon held together by weak van der Waals’ forces and low density. In the moon, the different layers experience less weight due to less gravitational force and the density is still reduced. The lighter layers can easily slide over another to make graphite more lubricating on the moon than on earth.
Question 3.
1. Identify the compound with the following structure:
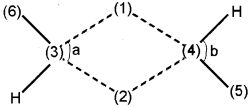
2. Mention the angles a & b.
3. Write down the corresponding elements in the figure.
1……………..
2……………..
3……………..
4……………..
5……………..
6……………..
Answer:
a) Diborane(B2H6).
b) a-97°
b-120°
c) 1-H, 2-H, 3-B, 4-B, 5-H, 6-H
Question 4.
The simplest boron hydride is diborane.
1. Write down the molecular formula of diborane.
2. Distinguish between terminal hydrogen and bridging hydrogen atoms of diboran.
Answer:
1. B2H6
2. In diborane, four hydrogen atoms and two boron atoms are present in a single plane. These hydrogen atoms are called terminal atoms. The terminal B-H bonds are regular two centre-two electron bonds. The remaining two hydrogen atoms above and below this plane are called bridging hydrogen atoms. The two bridge bonds (B-H-B) are three centre-two electron bonds.
Question 5.
1. How is orthoboric acid prepared?
2. Account for the acidic nature of orthoboric acid.
Answer:
1. Orthoboric acid is prepared by acidifying an
aqueous solution of borax.
Na2B4O7 + 2HCl + 5H2O → 2NaCl + 4B(OH)3
2. Orthoboric acid is a weak nonobasic acid. It acts as a Lewis acid by accepting electrons from a hydroxyl ion.
B(OH)3 + 2HOH → [B(OH)3]– + H3O+
Question 6.
1. How is diborane prepared in the laboratory?
2. BCl3 is a good Lewis acid. Why?
Answer:
1. Diborane can be conveniently prepared in the laboratory by the oxidation of sodium borohydride with iodine.
2NaBH4 + l2 → B2H6 + 2Nal + H2
2. In BCl3, the central boron atom contains only six electrons. Hence, it has the tendency to accept electrons and acts as a Lewis acid.
Question 7.
1. Name the allotropes of carbon.
2. Carbon monoxide is highly poisonous. Do you agree? Justify.
Answer:
1. Graphite, diamond and fullerene.
2. I agree with this statement. Because, CO has strong and reversible binding with haemoglobin resulting in the formation of carboxyhaemoglobin. This reduces the amount of haemoglobin available in blood for oxygen transport. This causes laboured respiration, muscle weakness and even death.
Question 8.
1. Diamond is hard and non conducting while graphite is soft and conducting. Why?
2. Explain the action of heat on boric acid.
3. What is inorganic benzene? How is it formed?
Answer:
1. In diamond, all the carbon atoms are in sp³ hybridised state. Due to this closely packed arrangement, it is hard. Since, there are no free electrons it is an insulator. But in graphite, the carbon atoms are in sp² hybridised state. It is a good conductor due to the presence of delocalised electrons between the layers. Graphite cleaves easily between the layers. Therefore, It is very soft and slippery.
2. On heating, orthoboric acid above 370 K forms metaboric acid, HBO2 which on further heating yields boric oxide, B2O3.
![]()
3. B3N3H6 is called Inorganic benzene. It is obtained by heating diborane with ammonia.
Question 9.
1. Explain the difference in properties of diamond and graphite on the basis of their structures.
2. How do you explain the lower atomic radius of gallium as compared to aluminium?
Answer:
1. In diamond, all the carbon atoms are in sp³ hybridised state. As a result of this hybridisation, a closely packed arrangement is present in diamond and it explains the hardness of diamond. Due to the absence of electrons, diamond is an insulator.
In graphite, the carbon atoms are in sp² hybridised state. As a result of this hybridisation, graphite has a layered structure with hexagonal rings. Each layer can slide over the other which explains the lubricating property of graphite.
2. This can be explained on the basis of the variation in the inner core of the electronic configuration. The presence of additional 10 d-electrons offer only poor screening effect for the outer electrons from the increased nuclear charge in gallium. Consequently, the atomic redius of gallium (135 pm) is less than that of aluminium (143 pm).
Question 10.
1. Boron resembles silicon in many of its properties. What is this resemblance generally known as?
2. What is dry ice? What is it used for?
3. What are silicones? How do they differ from silicates?
Answer:
1. Diagonal relationship
2. Carbon dioxide can be obtained as a solid in the form of dry ice, by allowing the liquified CO2 to expand rapidly.
Dry ice is used as a refrigerant for ice-cream and frozen food.
3. Silicones are a group of organosilicon polymers containing R2SiO repeating units.
Silicates are minerals with SiO44- as the basic structural unit. In silicates either the discrete unit is present or a number of such units are joined together via corners by sharing 1, 2, 3 or 4 oxygen atoms per silicate units.
Plus One Chemistry The p Block Elements Four Mark Questions and Answers
Question 1.
Match the following:
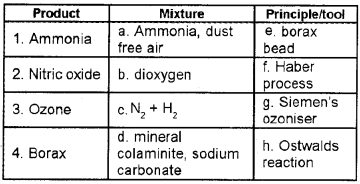
Answer:
1 – c – f
2 – a – h
3 – b – g
4 – d – e
Question 2.
a) Elements of group 13 are
1) Al, Cr, Cd, Ga, Ti
2) B, C, Si, Ga, Ti
3) B, Al, Ga, Ti, In
4) B, Al, Cr, Ca, Ti
b) Which of the following is not a mineral of boron? (borax, bauxite, colemanite, tincal)
c) In the reaction between NH3 and BF3 ammonia acts as
(Lewis base, Lewis acid, brownsted base)
d) Diamond and Graphite are carbon’s
(isotropic forms, allotropic forms, isotopic forms, amorphous form)
Answer:
a) 3) B, Al, Ga, TI, In
b) Bauxite
c) Lewis base
d) allotropic forms
Question 3.
The hydrides of boron are called boranes.
1. How diborane reacts with ammonia?
2. Account for the exceptional hardness of diamond.
Answer:
1. Diborane reacts with ammonia to give B2H6.2NH3
initially, which can be formulated as [BH2(NH3)2]+[BH4]–. Further heating gives borazine, B3N3H6 known as ‘inorganic benzene’ in view of its ring structure with alternate B-H and N-H groups,
2. In diamond, all the carbon atoms are in sp3 hybridised state. As a result of this hybridisation, a rigid three-dimensional network of carbon atom is generated with directional covalent bonds throughout the lattice. It is very difficult to break this network structure.
Plus One Chemistry The p Block Elements NCERT Questions and Answers
Question 1.
Why does boron trifluoride behave as a Lewis acid? (2)
Answer:
The B atom in BF3 has only 6 electrons in the valence shell and thus needs two more electrons to complete its octet. Therefore, it easily accepts a pair of electrons from nucleophiles such as F–, NH3, (C2H5)2O, RCH2OH etc. and thus behaves as a Lewis acid.
Question 2.
Explain why is there a phenomenal decrease in ionisation enthalpy from carbon to silicon? (2)
Answer:
Due to increase in atomic size and screening effect the force of attraction of the nucleus for the valence electron decreases considerably in Si as compared to C. As a result, there is a phenomenal decrease in ionisation enthalpy from carbon to silicon.
Question 3.
Consider the compounds, BCl3 and CCl4. How will they behave with water? (2)
Answer:
The B atom in BCl3 has only six electrons in the valence shell and hence is an electron-deficient molecule. It easily accepts a pair of electrons donated by water and hence BCl3 undergoes hydrolysis to form boric acid (H3BO3) and HCl.
BCl3 + 3H2O → H3BO3 + 3HCl
In contrast, C atom in CCl4 has 8 electrons in the valence shell. Therefore, it is an electron-precise molecule. As a result, it neither accepts nor donates a pair of electrons. In simple words, it does not accept a pair of electrons from H2O molecule and hence CCl4 does not undergo hydrolysis in water.
Question 4.
Aluminium trifluoride is insoluble in anhydrous HF but dissolves on addition of NaF. Aluminium trifluoride precipitates out of the resulting solution when gaseous BF3 is buddled through. Give reasons. (3)
Answer:
Anhydrous HF is a covalent compound and is strongly H-bonded. Therefore, it does not give free F– ions and hence AlF3 does not dissolve in HF. In contrast, NaF is an ionic compound and hence F– ions are easily available. As a result, it combines with AlF3 to form the soluble complex.
3NaF + AlF3 → Na3[AlF3]
On bubbling gaseous BF3, AlF3 is precipitated. It is because BF3 is a stronger Lewis acid than AlF3. This is attributed to smaller size and higher electronegativity of Boron. As a result of this B has much higher tending to form complexes than Al. Therefore, when BF3 is added to the above solution, AlF3 gets precipitated.
Na3[AlF6] + 3BF3 → 3Na[BF4] + AlF3(s)
Question 5.
In some of the reactions, thallium resembles aluminium, whereas in others it resembles with group 1 metals. Support this statement by giving some evidences. (3)
Answer:
Aluminium shows a uniform oxidation state of+3 in its compounds. Like aluminium, thallium also shows +3 oxidation state in some of its compounds like TlCl3, Tl2O3, etc. Al is known to form octahedral complexes like [AlF6]3-. Similarly, Tl also forms octahedral complexes as [TlF6]3-.
Thallium also resembles group 1 metals. Like group 1 metals which show a stable oxidation state of +1 in their compounds Tl, due to inert pair effect, also shows +1 oxidation state in some of its compounds such as Tl2O, TlCl, TlClO4, etc. Similarly, like group 1 oxides, Tl2O is strongly basic.
We hope the Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 11 The p-Block Elements help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 11 The p-Block Elements, drop a comment below and we will get back to you at the earliest.
