Plus One Chemistry Chapter Wise Questions and Answers Chapter 1 Some Basic Concepts of Chemistry is part of Kerala Plus One Chemistry Chapter Wise Questions and Answers. Here we have given Plus One Chemistry Chapter Wise Questions and Answers Chapter 1 Some Basic Concepts of Chemistry.
Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 1 Some Basic Concepts of Chemistry
Plus One Chemistry Some Basic Concepts of Chemistry One Mark Questions and Answers
Question 1.
Which of the following is a mixture?
a) Graphite
b) Sodium chloride
c) Distilled water
d) Steel
Answer:
d) Steel
Question 2.
1 µ g = __________ g
[10-3, 10-6, 10-9, 10-12] ‘
Answer:
10-6
Question 3.
The number of significant figures in 0.00503060 is __________ .
Answer:
6
Question 4.
The balancing of chemical equations is based on which of the following law?
a) Law of multiple proportions
b) Law of conservation of mass
c) Law of definite proportions
d) Gay-Lussac law
Answer:
b) Law of conservation of mass
Question 5.
Which among the following is the heaviest?
a) 1 mole of oxygen
b) 1 molecule of sulfur trioxide
c) 100 u of uranium
d) 44 g carbon dioxide
Answer:
d) 44 g carbon dioxide
Question 6.
Calculate the number of atoms in 48 g of He?
Answer:
Gram atomic mass of He = 4 g.
Thus, numberofatomsin4g (1 mol) He = 6.02 × 1023
So number of atoms in 48 g of He = × 6.02 × 1023
=12 × 6.02 × 1023
= 7.224 × 1024
Question 7.
One mole of CO2 contains how many gram atoms?
Answer:
3 gram atoms.
Question 8.
The ratio of gram atoms of Au and Cu in 22ct gold is __________
Answer:
7 : 2
Question 9.
A compound contains 69.5% oxygen and 30.5% nitrogen and its molecular weight is 92. The compound will be
Answer:
N2O4
Question 10.
The total number of electrons present in 1 mole of water is
Answer:
6 × 1024.
Question 11.
40g NaOH is present in 100 ml of a solution. Its molarity is __________
Answer:
10 M
Plus One Chemistry Some Basic Concepts of Chemistry Two Mark Questions and Answers
Question 1.
Classify the following substances into homogeneous and heterogeneous mixtures.
- Milk
- Iron
- Air
- Gasoline
- Kerosene
- Muddy water
Answer:
| Homogeneous | Heterogeneous |
| Milk, Iron Gasoline Air Kerosene | Muddy Water |
Question 2.
Calculate the volume occupied by 4.4 g of CO2 at STP?
Answer:
1 mole CO2 = 44 g
4.4 CO2 = 0.1 mole CO2
Volume occupied by 1 mol CO2 at STP = 22.4 L
∴ Volume occupied 0.1 mol CO2 at STP = 0.1 × 22.4 L
= 2.24 L
Question 3.
During a group discussion a student argued that “the water of sea and river should have different chemical composition”.
- What is your opinion?
- Which law would you suggest to support your answer?
- State the law.
Answer:
- I can’t join with him.
The water of sea and water of river must have the same chemical composition. - Law of definite proportions.
- A given compound always contains exactly the same proportion of elements by weight.
Question 4.
“When science developed some theories are also modified”.
Write the modified atomic theory.
Answer:
- Atom is no longer considered as indivisible, it has been found that atom is made up of sub atomic particles called protons, neutrons and electrons.
- Atoms of the same element may not be similar in all respects.
- Atoms of different elements may be similar in one or more respects.
- The ratio in which atomic unit may be fixed and integral but may not be simple.
- The mass of atom can be changed into energy.
Question 5.
Carbon combines with oxygen to form CO and CO2.
- What is the law behind this?
- State the law.
Answer:
- Law of multiple proportions.
- If two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
Question 6.
Calculate the volume occupied by 6.02×1025 molecules of oxygen at STP.
Answer:
Volume occupied by 1 mole of oxygen gas at STP = 22.4 l
i.e., Volume occupied by 6.02×1023 molecules of oxygen gas at STP = 22.4 l
Hence the volume occupied by 6.02 × 1025 molecules
of oxygen gas at STP = = 2240 l.
Question 7.
Calculate the molality of a solution of NaOH containing 20g of NaOH in 400 g solvent.
Answer:

Question 8.
Calculate the mole fraction of NaOH in a solution containing 20 g of NaOH per 360 g of water.
Answer:

Question 9.
12 g of carbon reacts with 32 g of oxygen to form 44g of carbon dioxide.
- Which law of chemical combination is applicable here?
- State the law.
Answer:
- Law of conservation of mass.
- Matter can neither be created nor destroyed. Or, during any physical or chemical change, the total mass of the products remains equal to the total mass of the reactants.
Question 10.
When hydrogen and oxygen combine to form water, the ratio between volume of reactants and products is 2:1:2.
- Which law of chemical combination is applicable here?
- State the law.
Answer:
- Gay Lussac’s law of gaseous volumes.
- When gases combine or are produced in a chemical reaction they do so in a simple ratio by volume provided all gases are at same temperature and pressure.
Question 11.
Carbon form two oxides, the first contains 42.9% C and the second contains 27.3% carbon. Show that these are in agreement with the law of multiple proportions.
Answer:
In the first compound:
C = 42.9%
O = 100-42.9 = 57.1%
So, the ratio between the masses of C and O = 42.9:57.1 = 1:1.33
In the second compound:
C = 27.3%
O= 100-27.3= 72.7%
So, the ratio between the masses of C and O = 27.3 : 72.7= 1:2.66
Hence, the ratio of masses of oxygen which combines with a fixed mass of carbon is 1.33:2.66 or 1:2, a simple whole-number ratio. This illustrates the law of multiple proportions.
Question 12.
Match the following:
| A | B |
| 1 amu | 1.008 x 1.66 x10–24 |
| Mass of 1 H atom | 6.02 x 1023 |
| Molar volume of O2 at STP | 11.2L |
| Volume of 14g of N2 at STP | 1.66 x 10–24 |
| Avogadro number | 22.4L |
Answer:
| A | B |
| 1 amu | 1.66 x 10–24 |
| Mass of 1 H atom | 1.008 x 1.6 x10–24 |
| Molar volume of O2 at STP | 22.4L |
| Volume of 14g of N2 at STP | 11.2L |
| Avogadro number | 6.02 x 1023 |
Question 13.
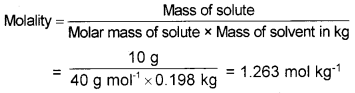
Calculate the molality of a solution containing 10 g ofNaOH in 200 cm3 of solution. Density of solution is 1.4 g/mL. (Molar mass of NaOH = 40)
Answer:
Mass of the solution = 200 × 1.04 = 208 g
Mass of NaOH (WB) = 10g Molar mass of NaOH (MB) = 40 g mol-1
Mass of water (WA) = (208 -10) g = 198 g = 0.198 kg
Question 14.
Calculate the mass percentage of oxygen in CaCO3.
Answer:
Molecular mass of CaCO3 = 100 g mol-1
Mass of oxygen in 100 g CaCO3=3 × 16 g = 48 g
Percentage of oxygen in CaCO3 =×100 = 48%
Question 15.
KCIO3 on heating decomposes to KCI and O2. Calculate the mass and volume of O2 produced by heating 50 g of KCIO3.
Answer:
The reaction is represented as,
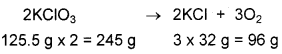
According to the equation, 96 g of oxygen is obtained from 245 g of KCIO3.
Hence mass of oxygen obtained from 50 g KCIO3 is
According to the equation 245 g of KCIO3 gives 3 moles of O2 at STP which is 3 × 22.4 L = 67.2 L
Volume of oxygen liberated by 50g of KCIO3
=
Question 16.
Calculate the number of molecules present in
- 11g of CO2.
- 56 mL Of CO2 at STP.
Answer:
1. = 0.25 mole
1 mole of CO2 contains 6.022 × 1023 molecules.
∴ 0.25 mole CO2 contains 6.022 × 1023 × 0.25
= 1.51 × 1023 molecules.
2. 56 mL = 0.056 L
=0.0025 mole
= 6.022 × 1023 × 0.0025=1.5 × 1021 molecules
Question 17.
Calculate the number of moles of 02 required to produce 240 g of MgO by burning Mg metal. [Atomic mass: Mg=24, 0=16]
Answer:
2 Mg + O2 → 2MgO
No. of moles of MgO = =6
No. of moles of 02 required = 6/2 = 3
Question 18.
Arrange the following in the increasing order of their mass.
(a) 1 g of Ca
(b) 12 amu of C
(c) 6.022 × 1023 molecules of CO2
(d) 11.2 L of N2 at STP
Answer:
a) Mass of 1 g Ca = 1 g
b) Mass of 12 amu C = = 2 × 10-23
c) Mass of 6.022 × 1023 molecules of CO2 = 44 g
d) Mass of 11.2 L of N2 at NTP = = 14 g
(b) < (a) < (d) < (c)
Question 19.
Complete the table:
| 42g N2 | 1.5 mole N2 | 33600mLN2 (STP) |
| 16g 0: | – – – – mole O2 | 11.2 L of O2 (STP) |
| ….g CO2 | 1 mole CO2 | – – – – L of C O2 (STP) |
| 28g CO | 1 mole CO | – – – – mLCO (STP) |
Answer:
| 42g N2 | 1.5 Mole N2 | 33600mLN2 (STP) |
| 16g O2 | 0.5 Mole O2 | 11.2 L of O2 (STP) |
| 44 g CO2 | 1 mole CO2 | 22.4 L of CO2 (STP) |
| 28g CO | 1 mole CO | 22400mLCO (STP) |
Question 20.
1. Irrespective of the source, pure sample of H20 always contains 88.89% by mass of oxygen and 11.11% by mass of hydrogen.
a) Which law is illustrated here?
b) State the law.
2. Complete the table by filling in the blanks:
| 48 g O2 | 1.5 mol O2 | ……mL O2 (at STP) |
| …… g Na | 2 gram atom Na | 2NA Na atoms |
| …….g CO2 | 2.5 mol CO2 | 56 L (at STP) |
| 8.5 g NH3 | ……mol NH3 | 11.2 L (at STP) |
Answer:
1. a) Law of definite proportions.
b) The same chemical compound always contains the same elements combined in the same fixed proprotion by mass.
2.
| 48 g O2 | 1.5 mol O2 | 33600 mL O2 (at STP) |
| 46g Na | 2 gram atom Na | 2Na Na atoms |
| 110 g CO2 | 2.5 mol CO2 | 56 L (at STP) |
| 8.5 g NH3 | 0.5 mol NH3 | 11.2L(atSTP) |
Question 21.
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction:
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
What mass of CaCO3 is required to react completely with 25 mL of 0.75 M HCl?.
Answer:
Mass of HCI in 0.25 mL 0.75 M HCl
As per reaction 100 g CaCO3 reacts with 2 × 36.5 = 73 g of HCl
∴ Mass of CaCO3 reacting with 0.6844 g HCl
Plus One Chemistry Some Basic Concepts of Chemistry Three Mark Questions and Answers
Question 1.
During a Seminar, a student remarked that “Dalton’s atomic theory has some faulty assumptions”.
a) Do you agree with him?
b) What is the present status of Dalton’s atomic theory?
c) Write any two wrong postulates of Dalton’s atomic theory.
Answer:
a) I agree with him. Out of 6 Dalton’s postulates, 5 postulates are faulty and only one is correct.
b) Dalton’s atomic theory has undergone many modifications.
c)
- All substances are made up of small indivisible particles called atoms.
- Atoms of the same element are identical in mass and other properties.
Question 2.
One gram atom of an element contains 6.023 × 1023 atoms.
- Find the number of atoms in 8 g oxygen.
- Which is heavier, 1 oxygen atom or 10 hydrogen atoms?
- Define mole and Avogadro number.
Answer:
1. 16 g oxygen contains 6.022 × 1023 atoms
∴ 8 g oxygen contains = 3.011 × 1023
2.
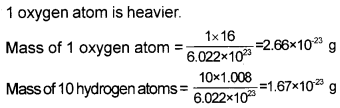
3. One mole is the amount of a substance that contains as many particles or entities as there are atoms in exactly 12 g (or 0.012 kg) of the 12C isotope.
Avogadro number – It is the number of discrete particles present in 1 mole of any substance. (Avogadro number, NA = 6.022 × 1023)
Question 3.
- Classify the following as homogeneous and heterogeneous mixtures.
Air, Smoke, Gunpowder, NaCI solution, Petrol, Bronze, Mixture of sugar and sand. - State and explain law of multiple proportions with example.
Answer:
1. Homogeneous-Air, NaCI solution, Bronze, Gun powder, Petrol.
Heterogeneous – Mixture of sugar and sand, Smoke.
2. When two elements combine to form more than one compound the different masses of one of the elements combining with fixed mass of the other element bear a simple ratio. ,
eg. Carbon reacts with oxygen to form two compounds viz. CO and CO2. In CO mass ratio is 12:16. In CO2 mass ratio is 12:32. Then mass ratio between oxygen in the 2 compounds is 16:32 or 1:2 which is a simple whole number ratio. Hence, the law is verified.
Question 4.
1. One mole of an ideal gas occupies 22.4 L at STP
a) Calculate the mass of 11.2 L of oxygen gas at STP.
b) Calculate the number of atoms present in the above sample.
2. 21 g of nitrogen gas is mixed with 5 g of hydrogen gas to yield ammonia according to the equation.
N2 + 3H2 → 2NH3
Calculate the maximum amount of ammonia that can be formed.
Answer:
1. a) Mass of 22.4 L oxygen at STP = 32 g
∴ Mass of 11.2 L oxygen at STP = 16 g
b) No. of atoms present in 16 g of O2
×2 = 6.02 × 1023 atoms
2. N2 + 3H2 → 2NH3
1 mole N2 + 3 mole H2 → 2moles NH3
1 mole N2 requires 3 mol H2
i.e., 28g N2 requires 6 g H2
Hence, 21 g N2 requires = 4.5 g H2
21 g N2 reacts completely and 0.5g H2 remains unreacted.
Hence, N2 is the limiting reagent.
28g N2 gives 2 × 17 g NH3
∴ 21 g N2 gives = 25.5 g NH3
Question 5.
When two elements combine to form more than one compound the different masses of one of the elements combining with fixed mass of the other bear a simple ratio.
i) Name the above law.
ii) Explain the above law by taking oxides of carbon.
Answer:
i) Law of multiple proportions.
ii) Carbon reacts with oxygen to form two compounds viz. CO and CO2.
In CO, mass ratio is 12:16
In CO2,mass ratio is 12:32
Ratio of the masses of oxygen combining with a fixed mass of carbon in the two compounds is 16:32 or 1:2, which is a simple whole-number ratio.
Question 6.
A compound contains 80% carbon and 20% hydrogen. If the molecular mass is 30 calculate the empirical formula and molecular formula.
Answer:
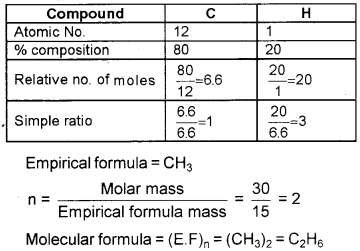
Question 7.
A compound contains 4.07% of hydrogen, 24.27% of carbon and 71.65% of chlorine. The molar mass is 98.96. What is the empirical and molecular formula?
Answer:
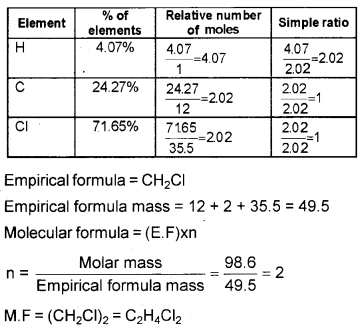
Question 8.
Nitrogen forms various oxides.
1. Identify the law of chemical combination illustrated here. Also state the law.
2. Determine the formula of each oxide from the given data and illustrate the law.

Answer:
1. Law of multiple proportions.
When two elements combine to form more than one compound the different mass of one of the elements which combine with the fixed mass of the other element bear a simple ratio.
2.

In NO and NO2, the masses of oxygen combining with a fixed mass (14 g) of nitrogen are in the ratio, 16:32 = 1:2. Similarly, in N2O and N2O3, the masses of oxygen combining with a fixed mass (28 g) of nitrogen are in the ratio, 16:48 = 1:3. These are simple whole-number ratios. Hence, the law of multiple proportions is verified.
Plus One Chemistry Some Basic Concepts of Chemistry Four Mark Questions and Answers
Question 1.
Which of the following weighs more?
a) 1 mole of glucose
b) 4 moles of oxygen
c) 6 moles of N
d) 5 moles of sodium
Answer:
a) 1 mole glucose = (72 + 12 + 96) g = 180 g
b) 4 moles of oxygen = 4 × 32g = 128g
c) 6 moles of nitrogen = 6 × 14 g = 84 g
d) 5 moles of Na = 5 × 23 g = 115 g.
Thus, 1 mole glucose weighs more.
Question 2.
3 g of H2 is mixed with 29 g of O2 to yield water.
1. Which is the limiting reagent?
2. Calculate the maximum amount of water that can be formed.
3. Calculate the amount of the reactants which remains unreacted.
Answer:
1.
![]()
According to the equation, 4 g H2 requires 32 g
So 3 g H2 requires = 24 g O2.
Here 3 g H2 is mixed with 29 g of O2. All H2 will react. Hence H2 is the limiting reagent.
2. According to the equation, 4 g H2 gives 36 g H2O. Hence 3 g H2 will give 36 × 3/4 = 27 g H2O.
3. Amount of O2 unreacted = (29 – 24)g = 5 g
Question 3.
a) Calculate the mass of oxygen required for the complete burning of 2 g of carbon.
b) Calculate the molar mass of (i) CO2 (ii) CH4
Answer:
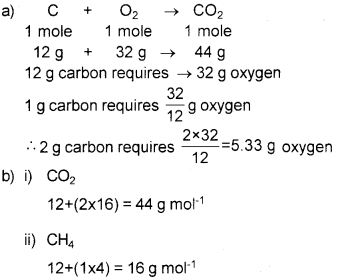
Question 4.
One gram mole of a substance contains 6.022 x 1023 molecules.
1. 24 g of carbon is treated with 72 g of oxygen to form CO2. Identify the limiting reagent.
2. Find the number of molecules of CO2 formed in this situation.
Answer:
1.
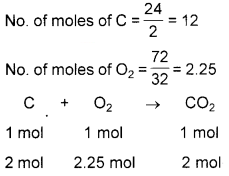
2 mol of C requires 2 mol of O2.
2 mol C completely reacts with 2 mol of O2 and 0.25 mol O2 and 0.25 mol O2 remains unreacted. Hence, C is the limiting reagent.
2. No. of moles of CO2 formed = 2
∴ No. of molecules of CO2 formed
= 2 × 6.022 × 1023 = 1.2044 × 1024
Question 5.
One gram mole of a substance contains 6.022×1023 molecules.
i) Find out the number of molecules in 2.8 g of nitrogen.
ii) Which is the heavier-one SO2 molecule or one CO2 molecule?
Answer:
i) No. of molecules in1 mole of N2 = 6.022 × 1023
i.e., No. of molecules in 28 g of N2 = 6.022 × 1023
∴ No. of molecules in 2.8 g N2
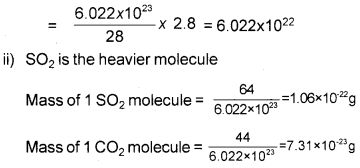
Question 6.
a) How can you illustrate the law of multiple proportions by using oxides of metals containing 78.7% and 64.5% of the metal?
b) Match the following:
1/12th the mass of C12 atom – 1 mole
1 g of hydrogen atom – amu
22.4 L O2 at NTP – gram mole
180 g of glucose – gram atom
6.022 × 1023 particles – molar volume
Answer:
a) In 100 g samples of the two oxides, the masses of the metal are 78.7 g and 64.5 g respectively.
First Oxide :
Mass of oxygen = 100 – 78.7 = 21.3 g
No. of parts by mass of oxygen combining with one part by mass of metal =
Second oxide:
Mass of oxygen = 100 – 64.5 = 35.5 g
No. of parts by mass of oxygen combining with one part by mass of metal =
The ratio of masses of oxygen combining with a fixed mass of metal = 3.7 : 1.9 = 2: 1, a simple whole number ratio.
b) 1/12th the mass C12 atom – amu
1 g of hydrogen atom – gram atom
22.4 L O2 at NTP – molar volume
180 g of glucose – gram mole
6.022 × 1023 particles – 1 mole
Question 7.
Calculate
1. The number of molecules present in 1 g of water.
2. The volume of 0.2 mole of sulphur dioxide at STP.
Answer:
1. Number of moles in 1 g water =
∴ No. of molecules in 1 g water
2. Volume of 0.2 mol S02 at STP = 0.2 × 22.4 litre
= 4.48 litre
Question 8.
“One mole of all substances contain the same number of specified particles.”
a) Justify the statement.
b) Howto connect mole, gram mole, and gram atom?
c) What is the relation between number of moles and volume?
d) Calculate the number of moles of a gas in 11.2 L at • STP.
Answer:
a) This statement is true i.e., one mole of all sub-stances contain the same number of specified particles. According to Avogadro’s law.
1 mole of any substance contains 6.022 × 1023 specified particles.
b)
![]()
1 gram mole is the molecular mass expressed in gram. It is the mass of 1 mole molecules in gram. Thus, 1 gram mole contains 1 mole molecules.
1 gram atom is the atomic mass expressed in gram. It is the mass of 1 mole atoms in gram. Thus, 1 gram atom contains 1 mole atoms.
c) Number of moles is directly proportional to volume (according to Avogadro law).
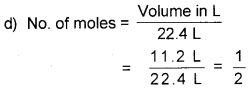
Plus One Chemistry Some Basic Concepts of Chemistry NCERT Questions and Answers
Question 1.
Calculate the molecular mass of the following : (3)
1. H2O
2. CO2
3. CH4
Answer:
1. Molecular mass of H2O = 2(1.008 u) +16.00 u
= 18.016u
2. Molecular mass of CO2 = 12.01 u + 2(16.00 u)
= 44.01 u
3. Molecular mass of CH4 = 12.01 u + 4(1.008 u)
= 16.042 u
Question 2.
Calculate the mass percent of different elements present in sodium sulphate (Na2SO4). (2)
Answer:
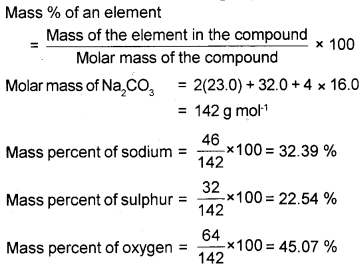
Question 3
Calculate the amount of carbon dioxide that could be produced when (3)
1. 1 mole of carbon is burnt in air.
2. 1 mole of carbon is burnt in 16 g of dioxygen.
3. 2 moles of carbon are burnt in 16 g of dioxygen.
Answer:
1. The balanced equation for the combustion of carbon dioxide in dioxygen in air is

In air combustion is complete.
Hence, Amount of CO2 produced when 1 mole of carbon is burnt in air = 44 g
2. As only 16 g dioxygen is available it is the limiting reagent.
Hence, amount of CO2 produced = ×16 = 22 g
3. Here again, dioxygen is the limiting reactant. Therefore, amount of CO2 produced from 16 g dioxygen = ×16 = 22g
Question 4.
Chlorine is prepared in the laboratory by treating manganase dioxide (MnO2) with aqueous hydrochloric acid according to the reaction,
4HCl(aq) + MnO2(s) → 2H2O(l) + MnCl2(aq) + Cl2(g)
How many grams of HCl react with 5.0 g of manganese dioxide?
(Atomic mass of Mn = 54.94 u) (2)
Answer:
1 mol of MnO2, i.e., 54.94 + 32 = 86.94 g MnO2 react with 4 moles of HCl, i.e., 4 × 36.5 g = 146 g of HCl.
∴ Mass of HCl reacting with 5.0 g of MnO2
Question 5.
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040. (2)
Answer:
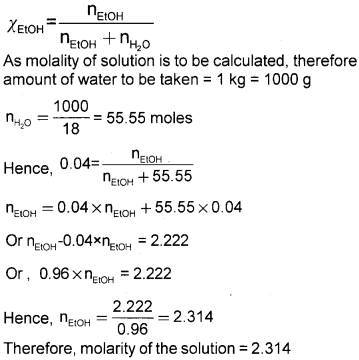
We hope the Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 1 Some Basic Concepts of Chemistry help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Questions and Answers Chapter 1 Some Basic Concepts of Chemistry, drop a comment below and we will get back to you at the earliest.
