Plus One Chemistry Chapter Wise Previous Questions Chapter 5 States of Matter is part of Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answer. Here we have given Plus One Chemistry Previous Questions Chapter 5 States of Matter.
Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 5 States of Matter
Question 1.
Mercury drops are spherical in shape. (March – 2009)
a) Which property is responsible for the spherical shape of drops? Explain the property.
b) How is the above property depend on temperature?
Answer:
a) Surface tension : It may be defined as the amount of work that must be done to expand its surface by unit area.
b) Surface tension of a liquid decreases with increase in temperature.
Question 2.
The theory that attempts to explain the behaviour of gases is known as kinetic molecular theory. (March – 2010)
a) On the basis of this theory, explain the compressible nature of gases and the temperature dependence on kinetic energy.
b) Liquid drops assume a spherical shape. Why?
c) How does temperature influence the viscosity of a liquid?
Answer:
a) Gases consist of large number of identical I particles that so small and so far a part on the average the actual volume of the molecule is negligible in comparison to the empty space between them. They are considered as point masses.
This assumption explains the greater compressibility of gases.
On heating, the gases K.E. of the particle increases and these particles strike the wall of I the container more frequently thus exerting more pressure.
b) Liquid tends to minimize their surface area. The spherical surface has a small surface area. So liquid drops assume a spherical shape.
c) Viscosity of a liquid decreases with rise in temperature. They are inversely proportional.
Question 3.
It is found that real gases do not obey the ideal gas equation perfectly under all conditions. (Say – 2010)
a) Write the ideal gas equation and mention the terms.
b) Why do real gases deviate from ideal behaviour?
c) What are the conditions under which real gases approach ideal behaviour?
Answer:
a) PV=nRT
P → Pressure; V → Volume; R → Universal gas constant; T → Temperature; n → no. of molecules
b) We find that two assumptions of the kinetic theory do not hold good. These are
i) There is no force of attraction between the molecules of a gas.
ii) Volume of the molecules of a gas is negligibly small in comparison to the space occupied by the gas.
c) Only at high temperature and low pressure.
Question 4.
In the Celsius scale, the melting point of ice is 0°C. Another scale of temperature is based on absolute zero. (March – 2011)
a) Identify the scale
b) What is the volume of a gas at absolute zero of temperature?
c) Draw a graph showing the relationship between volume and temperature of an ideal gas at a constant pressure.
d) Consider a gas at 0°C. At what temperature will the volume be doubled if the pressure is kept constant?
Answer:
a) Kelvin Scale
b) Zeno
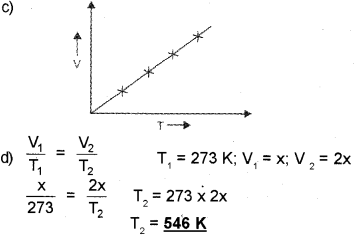
Question 5.
Consider the following isotherms of a gas: (Say – 2011)
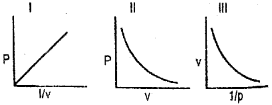
a) Which gas law is illustrated by these diagrams.
b) Draw the diagram when PV is plotted against P.
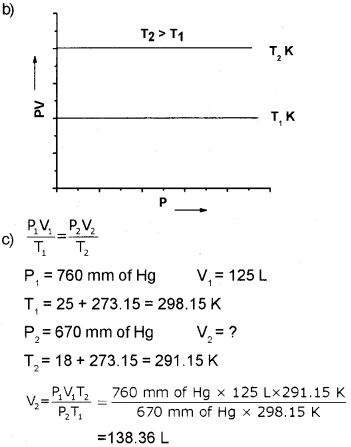
e) An air-filled balloon has a volume of 125L at a pressure of 760 mm of mercury and a temperature of 25°C. What will be its volume when the pressure is 670mm of mercury and temperature is 18°C?
Answer:
a) Boyles law.
Question 6.
Gas laws are relationships between the measurable properties of gases. (March – 2012)
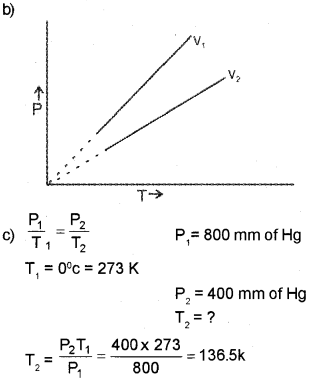
a) Name the gas law which gives the relationship between the pressure and temperature of a fixed amount of gas at a constant volume.
b) Draw the graph to illustrate the above gas law.
c) A definite quantity of an ideal gas is confined in a container of constant volume. When the container is immersed in a bath of melting ice, the pressure of the gas is 800 mm of Hg. Find the temperature when the gas pressure is 400 mm of Hg.
Answer:
a) gay-Lussac’s law
Question 7.
a) The combination of Boyle’s law, Charle’s law and Avogadro’s law is known as ideal gas equation. But the real gases deviate from ideal behaviour. (Say – 2012)
i) Write the modified ideal gas equation.
ii) Name the above equation.
b) Give the reason for the following:
i) At hill station, the pressure cooker is used for cooking.
ii) Window panes of the old building become thicker at the bottom than at the top.
Answer:
a) 1) Modified ideal gas equation.
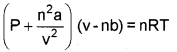
P → Pressure, v – volume
n-No. of moles of gas.
a, b → van der Waals’ constant,
b)
- The boiling point of water is low at Hill station.
- Glass is an amorphous substance.
Question 8.
Real gases behave ideally only at certain conditions. (March – 2013)
a) What is Boyle point of a gas?
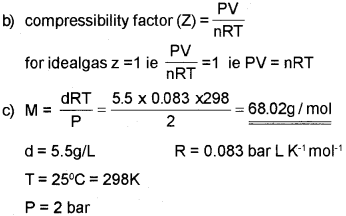
b) Write the expression for the compressibility factor. What is its value for an ideal gas?
c) Density of a gas was found to be 5.5 gL-1 at 2 bar pressure. Calculate its molar mass. (R = 0.083 bar L mof1 K-1).
Answer:
a) Boyle poin of a gas :- The temperature at which a real gas obeys ideal gas law over an appreciable range of pressure is called Boyle temperature (Tb) or Boyle point.
Question 9.
a) i) What is ther modynamic scale of temperature? (Say – 2013)
ii) Viscosity of liquids decreases as the temperature increases. Why?
iii) Gases deviate from ideal behaviour due to the faulty assumptions of the kinetic theory of gases. State those faulty assumptions.
OR
b) i) van der Waals’ equation of state explains the behaviour of real gases. What does the van der Waals’ constant ‘a’ indicate?
ii) What is the critical temperature of a gas?
iii) At 25°C and 760 mm Hg pressure a gas occupies 600mL volume. What will be its pressure at a height where the temperature is 10°C and volume of the gas is 640 mL?
Answer:
a) i) Kelvin scale
ii) Viscosity of liquids decreases as the temperature rises because at high-temperature molecules have high kinetic energy and can overcome the intermolecular forces to slip past one another between the layers.
iii)
- There is no force of attraction between the molecules of a gas.
- Volume of the molecules of a gas is negligibly small in comparison to the space occupied by the gas.
OR
b) i) The van der Waals’constant‘a’is a measure of the magnitude of intermolecular attractive forces within the gas and is independent of temperature and pressure.
ii) It is the temperature at which a gas and its vapour coexist and above which the gas cannot be liquified by application of external pressure.
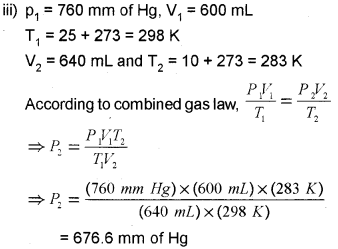
Question 10.
a) What is Boyle point or Boyle temperature? (March – 2014)
b) At high altitudes, a pressure cooker is used for cooking food. Why?
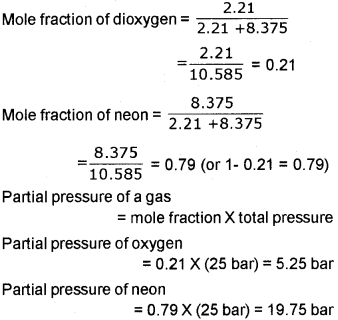
c) A neon-dioxygen mixture contains 70.6 g dioxygen and 167.5 g neon. If the pressure of the mixture of gases in the cylinder is 25 bar, what are the partial pressures of 02 and neon in the mixture?
OR
a) Particles of soil at the bottom of a river remain separated, but they stick together when taken out. Name the property behind this.
b) Critical temperatures of ammonia and C02 are 405.5 K and 304 K respectively. On cooling these gases from 500 K, which gas will liquify first?
c) van der Waals’ forces are attractive intermolecular forces. Write the names of any two types of vander Waals’ forces.
Answer:
a) It is the temperature at which a real gas obeys ideal gas law over an appreciable range of pressure.
b) At high altitudes atmospheric pressure is low. Therefore, liquids at high altitudes boil at lower temperatures in comparison to that at sea level. Since water boils at low temperature at high altitudes, the pressure cooker is used for cooking food.
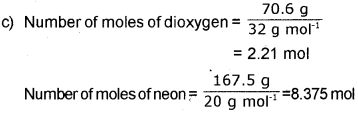
OR
a) Surface tension
b) Ammonia will liquify first because its critical temperature will be reached first. Liquefaction of carbon dioxide will require more cooling.
c) Dispersion forces or London forces, Dipole-Dipole forces.
Question 11.
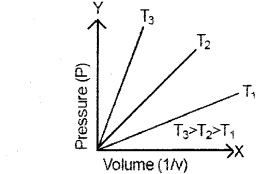
a) Name the gas law shown by the above graph. (August – 2014)
b) Statethegaslaw.
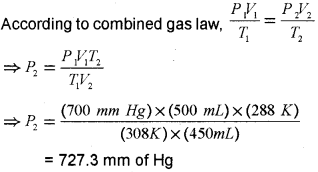
c) At 35°C and 700 mm of Hg pressure, a gas occupies a 500mL volume. What will be its pressure when the temperature is 15°C and the volume of the gas is 450m1?
Answer:
a) Boyle’s law.
b) At constant temperature, the pressure of a fixed amount of gas varies inversely with its volume.
Question 12.
The gases which obey Gas Laws at all temperatures and pressures are called ideal gases. (March – 2015)
a) Give reasons for the deviation of real gases from the ideal gas behaviour.
b) Calculate the minimum pressure required to compress 500 ml of air at 1 atm to 300 mL at the same temperature.
Answer:
a) Real gases deviate from ideal behaviour at high pressure and low temperature. At these conditions the two assumptions of the kinetic theory do not hold good, These are
- There is no force of attraction between the molecules of a gas.
- The volume of the molecules of a gas is negligibly small in comparison to the space occupied by the gas.
At high pressure and low-temperature gas molecules are close together and there is a force of attraction and repulsion between them. Also, at high pressure, the volume of the gas molecules becomes significant because these are restricted to a lower volume.

Question 13.
The kinetic molecular theory provides a theoretical basis to experimentally observed facts related to gases. (Say – 2015)
a) Which one of the following statements is CORRECT with regard to the gaseous state?
i) Molecules have fixed positions.
ii) Molecules are in constant random motion.
iii) All molecules have same speed at a given temperature.
iv) The average kinetic energy of the gas molecules is inversely proportional to the absolute temperature.
b) A sample of hydrogen gas occupies a volume of 300 ml at 1.2 bar pressure and 5°C. Calculate its volume at 0.45 bar pressure and 70°C.
Answer:
a) ii) Molecules are ¡n constant random motion.
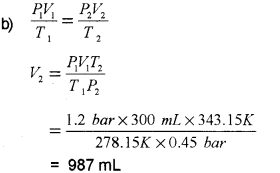
Question 14.
Ideal gas equation is tme for ideal gases only. There is a modified form of ideal gas equation applicable to all gases. (March – 2016)
a) Give the name of the modified form of ideal gas equation and write down it.
b) Name the phenomenon behind the cleansing action of soap.
c) What do you know about Dalton’s law of partial pressures?
Answer:
a) van derWaals’equation.
For n’ moles of a gas the equation is
![]()
b) Surface tension
c) The Dalton’s law of partial pressures states that the total pressure exerted by the mixture of non-reacting gases is equal to the sum of the partial pressures of individual gases.
Mathematically,
PTotal = p1 + p2 + p3 + …………… (at constant T, V)
where PTotal is the total pressure exerted by the mixture of gases and p1, p2, p3 etc. are the partial pressures of gases.
Question 15.
An ideal gas is one which obeys gas laws.
a) Derive an ideal gas equation.
b) AT 27°C a gas was compressed to half of its volume. To what temperature it must be heated, so that it would occupy double its original volume?
i) 54CC
ii) 327k
iii) 600 K
iv) 300 K
c) Liquid drops assume a spherical shape. Why?
Answer:
According to Boyle’s law, at constant T and n:
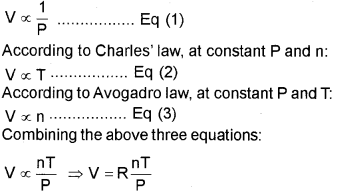
where R is a proportionality constant known as the universal gas constant.
OR PV = nRT, which is the ideal gas equation.
b) 1200 K (not in the options)
(Explanation: Le original volume of the gas = V
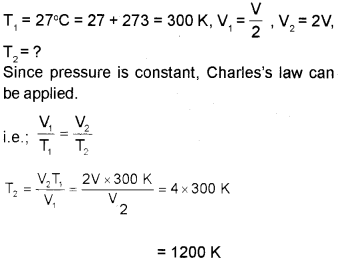
c) This is a consequence of surface tension. The lowest energy state of the liquid will be when the surface area is minimum. Spherical shape satisfies this condition. Hence liquid drops assume a spherical shape.
Question 16.
a) Give the reason behind the following. (March – 2017)
i) The glass window pannels of old buildings are thicker at the bottom than at the top.
ii) Sharp glass edges are heated for making them smooth.
b) Maxwell and Boltzmann have shown that actual distribution of molecular speeds depends on temperature and molecular mass.
i) What do you mean by most probable velocity?
ii) At the same temperature which will move faster, N2 or Cl2?
Answer:
a) i) Glass is an extremely viscous liquid. It is so viscous that many of its properties resemble solids. Thus, glass is considered as a pseudo solid. It has a tendency to flow very slowly. Hence, the glass window panes of old buildings are thicker at the bottom than at the top.
ii) On heating, the glass melts and the surface of the liquid tends to take the rounded shape at the edges due to surface tension, which makes the edges smooth.
b) i) Most probable velocity is the velocity possessed by the maximum number of gas molecules at a given temperature. It corresponds to the maximum of the Maxwell- Boltzmann distribution of molecular velocities,
ii) N2. (Explanation: At the same temperature gas molecules with lighter mass have greater speed than heavier gas molecules. Thus, at the same temperature light N2 molecules move faster than heavier Cl2 molecules.)
We hope the Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 5 States of Matter help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 5 States of Matter, drop a comment below and we will get back to you at the earliest.
