Plus One Chemistry Chapter Wise Previous Questions Chapter 3 Classification of Elements and Periodicity in Properties is part of Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answer . Here we have given Plus One Chemistry Previous Questions Chapter 3 Classification of Elements and Periodicity in Properties.
Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 3 Classification of Elements and Periodicity in Properties
Question 1.
a) Who introduced the periodic law of elements for the first time? State the law. (March – 2009)
b) State the modern periodic law of elements.
Answer:
a) Mendeleev’s Periodic law : The physical and chemical properties of elements are periodic functions of their atomic mass.
b) Modern Periodic law: The physical and chemical properties of elements are a periodic function of their atomic numbers.
Question 2.
Account for the following: (March – 2010)
a) Ionization enthalpy of nitrogen is greater than that of oxygen.
b) Atomic radius decreases from left to right in a period.
c) Electron gain enthalpy of F is less negative than that of Cl.
Answer:
a) Ionisation enthalpy of nitrogen is greater than that of oxygen. This arises because in nitrogen atom, three 2p electrons reside in different atomic orbitals. (Hund’s rule) whereas in the oxygen atom, two of the four2p electrons must occupy the same 2p orbitals resulting in an increased electron repulsion. Consequently, it is easier to remove the fourth 2p electrons from oxygen than it is, to remove one of the three 2p electrons from nitrogen.
b) The atomic size generally decreases across a period. It is because within the period the outer electrons are in the same valence shell and the effective nuclear charge increases as the atomic number resulting in the increased attraction of the electrons to the nucleus. So, atomic radius decreases from left to right.
c) Within a group electron gain enthalpy becomes less negative down a group. However, adding an electron to the 2p orbital leads to greater repulsion than adding an electron to the larger 3p or bital. Hence electron gain enthalpy of F is less negative that of Cl.
Question 3.
Development of Periodic Table have made the study of elements and their compounds easier. (Say – 2010)
a) Discuss about the main features of Mendeleev’s periodic table.
b) State the modem periodic law.
c) Give the name for the element with atomic number 112.
Answer:
a) Mendeleev’s periodic table made the study of chemistry of elements easier and systematic. He left vacant spaces in the original table for elementsto be discovered. From the position of the elements their properties can be understood.
b) The physical and chemical properties of the elements are periodic functions of their atomic number.
c) Ununbium
Question 4.
A graph of atomic radius versus atomic number is given below: (March – 2011)
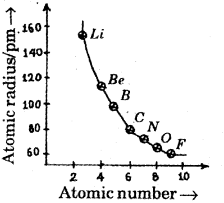
a) What do you understand from this graph?
b) Account for the observation that cations are always smaller than the parent atom while anions are always larger than the parent atom.
c) Using the above graph, how will you account for the variation of ionization enthalpy in a period?
Answer:
a) Atomic size decreases with increase in atomic number.
b) With loss of electrons or decrease in no. of shells, effective nuclear charge increases and hence size of the atom decreases, ie, the size of a cation is less than that of the atom. With addition of electrons, effective nuclear charge decreases and size increases.
c) Ionisation enthalpy decreases with increase in size of the atom.
Question 5.
a) A graph showing the variation of atomic Radius with atomic number for alkali metals is given below. (Say – 2011)
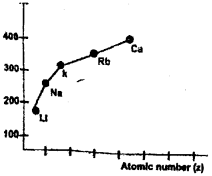
Comment on the variation of atomic radius with increase in atomic number in a group. Give reason.
b) What is meant by isoelectronic species
c) Select the isoelectronic species from the follow-ing n, O2, F, Mg2+, Al2+, Na+
Answer:
a) Atomic radii of alkali metals increases down the group with increase in atomic number. Down the group the principal quantum number (n) increases and the valence electrons are farther from the nucleus. This happens because the inner energy levels are filled with electrons, which serve to shield the outer electrons from the pull of the nucleus. Concequently the size of the atom increases as reflected in the atomic radii.
b) Isoelectronic species are atoms, ions or mol-ecules having same number of electrons.
c) O2-, F’, Mg2+ and Na+ are isoelectronic as they contain 10 electrons each.
Question 6.
Moseley modified Mendeleev’s periodic law based on his observation on the X-ray spectra of elements. (March – 2012)
a) State the modern periodic law.
b) The IUPAC name of the element with atomic number 109 is ………….
c) Analyze the following graph between ionization enthalpy and atomic number.
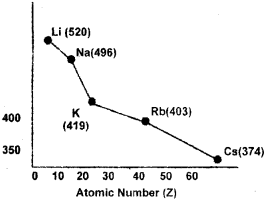
What do you observe from the graph? Give justi fication foryourobsérvation.
Answer:
a) Modern periodic Law: “The physical and chemical properties of elements are periodic functions of their atomic numbers.
b) 109 → Unnilennium.
c) The graph represents a variation of AH (Ionisation enthalpy) along a group.
On moving down the group, the size of atom increases and the outer electrons are far away from the nucleus. Also there is an increased screening of nuclear charge by the electrons in the inner shells. Thus the removal of electrons becomes easier down the group and ionisation enthalpy decreases.
Question 7.
a) Electron gain enthalpy is the amount of energy released when an isolated gaseous atom accepts an electron to form a monovalent anion. The values of electron gain enthalpy with atomic number of Halogens are given below: (Say – 2012)
| At. No. | Δeg H in kJ/mol | |
| F | 9 | 328 |
| Cl | 17 | 349 |
| Br | 35 | 325 |
| I | 53 | 295 |
i) Why electron gain enthalpy decreases from chlorine to Iodine?
ii) Chlorine has more electron gain enthalpy than Fluorine. Why?
b) Identify the largest and smallest ion given below: O2-, F–, Na+ and Mg2+
Answer:
a) 1) Electron gain enthalpy decreases from chlorine to iodine ie. less negative on moving down the group because atomic size increases.
2) Because of small size of flourine, fluorine cannot hold eight electron in its outermost shell due to very high inter electronic repulsion.
b) Largest ion – O2-
Smallest ion – Mg2+
| Ion | Ionic radii |
| O2– | 140 pm |
| F- | 125 pm |
| Na+ | 95 pm |
| Mg2+ | 65 pm |
Question 8.
The reactivity of an element is very much related to its ionization enthalpy. (March – 2013)
a) In general, ionization enthalpy increases from left to nght across a period. Give the reason,
b) Oberve the following graph in which the first ionization enthalpies (Δ1H) of elements of the second penod are plotted against atomic numbers (Z):
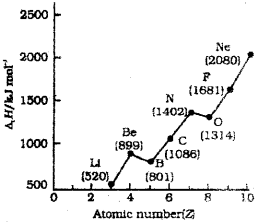
Identify the anomalous values and justify.
Answer:
a) From left to right in a period, the ionisation en thalpy of element increases. This is due to the increase in nuclear charge and decrease in atomic size from left to right in a period.
b) ‘Be’ has exceptionally high ionisation enthalpy than ‘B’ because ‘Be-has stable fully filled 2s2 electronic configuration.
‘N’ has high I. E then ‘O’ because ‘N’ has stable half filled 2p3 electronic configuration.
Question 9.
a) The IUPAC has made some recommendations to name elements with atomic numbers above 100. What would be the name for the element with atomic number 104? (Say – 2013)
b) Electron egativity is the ability of an element to attract a shared pair of electrons. Name a numencal scale of electronegativity of elements.
c) Give reason for the following:
i) Phosphorus forms PCI5 while nitrogen cannot from Nd5. Why?
ii) The first ionisation enthalpy of oxygen is smaller compared to nitrogen.
Answer:
a) Unnilquadium
b) Pauling Scale
c) i) Nitrogen, being a second period has no va cant d orbitais in its valence shell, it has only four orbitais in its valence shell (one 2s and three 2p). It cannot expand its covalency be yond four and hence cannot form pentahalides. But phosphorus has vacant d-orbitais in its valence shell. Hence it can expand its coya lency beyond four and forms penta halides.
ii) This arises because in nitrogen atom, three 2p-electrons reside in different atomic orbitais in accordance with Hund’s rule whereas in oxygen atom, two of the four 2p-electrons must occupy the same 2p-orbitai resulting in an in creased electron-electron repulsion. Conse quently it is easier to remove the fourth 2p-electron from oxygen that it is, to remove one of the three 2p-electrons from nitrogen. Hence, the first ionisation enthalpy of oxygen is smaller as compared to that of nitrogen.
Question 10.
a) Thefirst member of a group of elements in the s and block differs from the rest of the family in chemical behaviour. Write any one reason for this. (March – 2014)
b) The first ionIzation enthalpy of sodium is lower than that of magnesium but its second ionization enthalpy is higher than that of magnesium.
Explain.
c) Write the general outer electronic configuration of d-block elements.
Answer:
a) Small size /High ionisation enthalpy/Non-availability of d-orbitals.
b) For Na (Z = il, is2 2s2 2p6 3S1), the first electron is removed from 3s orbital to form Na which has the stable configuration of the nearest noble gas, He (is2 2s2 2p6). So further removal of electron requires higher energy. But for Mg (Z = 12, Is2 2s2 2p6 3s2), both electrons are to be removed from the 3s orbital to get the stable configuration of the nearest noble gas, He (Is2 2s2 2p6)
c) (n – 1)d1-10 ns°-2
Question 11.
a) Transition elements were placed in between group 3 and group 12 of the periodictable. Give any two characteristics of transition elements. (August – 2014)
b) Does the ionization enthalpy decrease along a group? Give reason.
Answer:
a)
- They mostly form coloured ions.
- They exhibit variable valence (oxidation states).
b) Yes
Down a group in the penodic table, the outermost eIecron being increasingly farther from the nucleus, there is an increased shielding of the nuclear charge by the electrons in the inner levels. Increase in shielding outweighs the increasing nuclear charge and the removal of the outermost electron requires less energy down a group.
Question 12.
Names of elements with atomic numbers greater than 100 are given by IUPAC. (March – 2015)
a) The atomic number of the element with IUPAC name ‘Ununbium’ is ………………….
i) 112
ii) 110
iii) 111
iv) 114
b) Why is potassium considered as an s block element?
c) The first ionization enthalpies of second-period elements generally increase from left to right along the period. Give reasons for this general trend.
Answer:
a) i) 112
b) Elements are classified into blocks depending on the type of atomic orbital in which the last electron enters. In potassium, the last electron enters into the s-orbital of the valence shell. Hence, it is considered as an s-block element.
c) From left to right across the second period, successive electrons are added to orbitais in the same principal quantum level and the shielding of the nuclear charge by the inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus. Thus, across a period, increasing nuclear charge outweighs the shielding. Conse quently, the outermost electrons are held more and more tightly and the ionization enthalpy increases.
Question 13.
Ionization enthalpy and atomic radius are closely related properties. (Say – 2015)
a) Analyse the following graph:
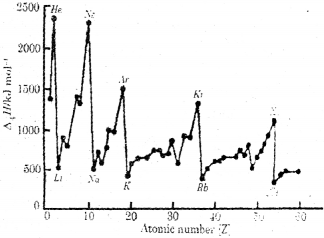
b) What conclusion can you derive from the graph regarding the first ionization ethalpies of alkali metals and noble gases? Justify your answer.
Answer:
a) In each respective period alkali metals have the least ionization enthalpy and noble gases have the maximum ionization enthalpy. Due to the closed electron shells and very stable electronic configuration of noble gases they have maximum ionization enthalpy values in each respective period.
In the case of alkali metals due to big atomic size and shielding effect of inner electrons there is weak attraction of electrons towards the nucleus and maximum repulsion of electrons from each other.
Hence, the effective nuclear charge experienced by the valence electron is less than the actual charge of the nucleus.
Hence, they have minimum ionization enthalpy in each respective period.
b) Aluminium belongs to third period and has vacant ‘d’ orbitais in its valence shell.
Hence it can expand its valence shell to accommodate more than four pairs of electrons. Boron, being a second period element has only four orbitais (one in 2s and three in 2p) and has no ‘d’ orbitaIs in its valence shell. As a consequence its maximum covalency is four.
Question 14.
a) Account for the following: (March – 2016)
i) Ionization enthalpy of Nitrogen is greater than that of Oxygen.
ii) 2nd period elements show anomalous behaviour.
b) A group of ions are given below. Find one pair which is NOT isoelectronic.
Na+, Al3, Ca2+, Br, F
Answer:
a. i) This arises because in nitrogen atom, three 2p-electrons reside in different atomic orbitaIs in accordance with Hund’s rule whereas in the oxygen atom, two of the four 2p-electrons must occupy the same 2p-orbital resulting in an in creased electron-electron repulsion.
Consequently, it is easier to remove the fourth 2p-electron from oxygen than it is, to remove one of the three 2p-electrons from nitrogen.
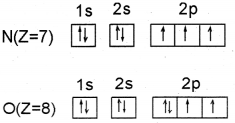
ii) The anomalous behaviour of second-period elements is attributed to their
1) small size
2) large charge/radius ratio
3) high electronegativity
4) only 4 valence orbitaIs (one in 2s and three in 2p) available for bonding
5) absence of vacant d-orbitaIs
6) greater ability to form p π – p π multiple bonds to itself and to other second period elements
b) Ca2 and Br (or any other non-iso electronic pairs such as
Na+ & Br–
AP+ & Br–
Ca2+ & F
Na+ & Ca2+
Al3+ & Ca2+
Br & F etc.)
Question 15.
a) In the periodic table, elements are classified into four blocks, Explain any two blocks.
b) Accountforthefollowing:
i) First ionization enthalpy of Boron is less than that of carbon.
ii) First member of a group differs from the rest of the members of the same group.
Answer:
a) The elements are classified into four blocks viz., s-block, p-block, d-block and f-block depending on the type of atomic orbitais that are being filled with electrons.
1) The s-block elements : The elements of Group 1 (alkali metals) and Group 2 (alkaline earth metals) which have ns1 and ns2 outermost electronic configuration belong to this block. They are all reactive metals with low ionization enthalpies.
The lose the outermost electron(s) readily to form 1+ ion (Inthe case of alkali metals) and 2+ ion (in the case of alkaline earth metals). The metallic character and the reactivity increase down the group. Because of high reactivity they are never found pure in nature: The compounds of s-block element are predominantly ionic (except compounds of Li and Be).
2) The p-block elements : This block comprises those elements belonging to Group 13 to 18. The outermost electronic configuration vanes from ns2np1 to ns2 np6 in each period. At the end of each period is a noble gas element with a closed valence shell ns2 np6 configuration.
This configuration is highly stable and hence noble gases exhibit very low chemical reactivity. Preceeding the noble gases are two chemically important groups of non-metals such as halogens (Group 17) and the chalco gens (Group 16).
The non-metallic character increases from left to right across a period and metallic character increases down the group.
3) The d-block elements (Transition Elements): These are the elements of Group 3 to 12 in the centre of the Periodic Table, which are chracterised by the filling of innerd- orbitals. They have the general outer electronic configuration (n – 1)d1-10 ns1-2.
They are all metals and mostly form coloured ions, exhibit variable valence and paramagnetism and are used as catalysts. The transition elements form a bridge between the chemically active metals of s-block and less active elements of Groups 13 and 14.
4) The f-block elements (Inner-Transition Elements): This include two rows of elements at the bottom of the Periodic Table such as Lanthanoids and Actinoids. These are characterised by the outer electronic configuratin (n – 2)f1-14 (n – 1)d0-1 ns2. The last electron added to each element is filled in f- orbital.
They are all metals. Within each series, the properties of the elements are quite similar. The chemistry of actinoids is more complicated due to large number of possible oxidation states. Actinoids are radioactive.
The elements after uranium are called transuranium elements. (Explanation of any two blocks is required)
b) i) Ionization enthalpy increases from left to right across a period due to decrease in atomic size and increase in effective nuclear charge. Carbon has high first ionization enthalpy than boron due to its small atomic size and high effective nuclear charge.
ii) The first element of each of the groups 1 and 2 and groups 13 -17 differs in many respects from the other members of their respective group.
This anomalous behaviour is attributed to their small size, high ionization enthalpy, large charge/radius ratio, high electro negativity, non-availability of d-orbitals and greater ability to form p π – p π multiple bonds to itself and to other second period elements (in the case of first members of p-block elements).
Question 16.
Electron gain enthalpy is one of the important periodic property. (March – 2017)
a) Define electron gain enthalpy.
b) Explain any two factors affecting electron gain enthalpy.
c) Write the oxidation state and co-valency of Al in (Al F6)3-.
Answer:
a) Electron gain enthalpy (ΔegH) is defined as the enthalpy change accompanying the addition of an electron to a neutral gaseous atom in its ground state to convert it into a negative ion.
b) The factors affecting electron gain enthalpy are atomic size, effective nuclear charge, shielding effect, electronic configuration, etc.
Atomic size – as atomic size increases the added electron would be farther from the nucleus and ΔegH decreases, i.e., becomes less negative. Effective nuclear charge – As effective nuclear charge increases it will be easier to add an electron because it will be closer to the nucleus. Thus, ΔegH increases, i.e., becomes more negative.
Shielding effect – as shielding effect increases effective nuclear charge decreases, attraction between the added electron and nucleus decreases and hence ΔegH decreases, i.e., becomes less negative.
Electronic configuration – if the atom has stable electronic configuration (octet, completely filled and half-filled configurations) ΔegH will be less, i.e., less negative, (any two factors required)
c) The oxidation state of Al is +3 (Al3+) and the co-valency is 6.
We hope the Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 3 Classification of Elements and Periodicity in Properties help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 3 Classification of Elements and Periodicity in Properties, drop a comment below and we will get back to you at the earliest.
