Plus One Chemistry Chapter Wise Previous Questions Chapter 12 Organic Chemistry Some Basic Principles and Techniques is part of Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answer . Here we have given Plus One Chemistry Previous Questions Chapter 12 Organic Chemistry Some Basic Principles and Techniques.
Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 12 Organic Chemistry Some Basic Principles and Techniques
Question 1.
C2H6 and C5H12 are members of a homologous series. (February – 2008)
a) What is a homologous series?
b) What is the general molecular formula of the above homologous series?
c) What is the significance of CH2 group in homologous series?
Answer:
a) A series of similarly constituted organic compounds in which the members possess the same functional group and have similar chemical properties.
b) CnH2n+2
c) The neighbouring members differ by CH2 unit in their molecular formula.
Question 2.
a) Write the IUPAC names of the following compounds: (March – 2009)
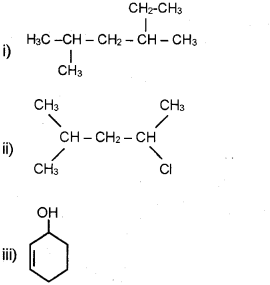
b) Draw the structure of the molecules represented by the IUPAC names, pent-4-ene-2-ol and 3- nitrocyclohexene.
Answer:
a) i) 2,4- Dimethylhexane
ii) 2-Chloro 4-methyl pentane
iii) Cyclohex-2-ene-1-ol
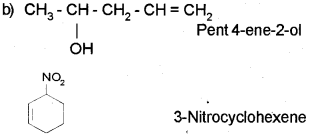
Question 3.
The bond line representation of cyclopropane is ![]() . (March – 2010)
. (March – 2010)
Write the bond line structures of
a) Cyclohexane
b) 2-Bromobutane
c) CH3CH(OH)CH2CHBrCH3
Answer:
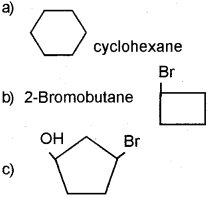
Question 4.
The IUPAC name of an organic compound is derived by identifying the functional group and the parent hydrocarbon chain.
a) Write the IUPAC names of the following.
b) Give the structures of the following compounds.
i) 3-Ethyl-4,4-dimethylheptane
ii) 6-Methyloctan-3-ol
Answer:
a) i) 2,2,4-Trimethyl pentane
ii) Hex-4-ene-1-oicacid
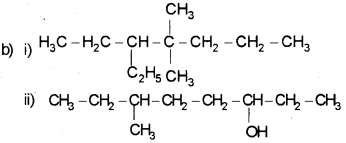
Question 5.
Detection of elements like nitrogen, halogens and sulphur are done using Lassigne’s test. Discuss the chemistry of Lassigne’s test forthe above elements. (Say – 2010)
Itm Detection of Nitrogen
The chemical reaction involve in the test for nitrogen is Na + C + N → NaCN
Fe2+ + 6CN → [Fe(CN)6]-4 (hexacyanoferrate (II))
In presence of con.H2SO4 some Fe2+ ions get oxidised to Fe3+ ions which react with hexacyanoferrate to form Iron hexacyanoferrate or femferrocyanide (blue)
3[Fe(CN)6]4- + 4Fe3- → Fe4 [Fe(CN)6]3 (Prussian blue)
In the case of compounds containing N and S the extract will contain sodium thiocyanate (NaCNS). This would give a blood red colour in presence of Fe ions.
Detection of Sulphur
If sulphur is present in the organic compound so dium sulphide is present in the Lassaigne’s extract.
2Na + S → Na2S
The extract is divide into two parts. To one portion of the extract add sodium nitroprusside. A purple colour indicate the presence of Sulphur.
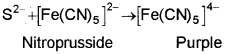
In the second portion, add dil. acetic acid and lead acetate. A black ppt is obtained.
S2+ + Pb2- → PbS (black)
Detection of halogens
The sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. A white precipitate, soluble in ammonium hydroxide shows the presence of chlorine, a yellowish precipitate sparingly soluble ¡ri ammonium hydroxide shows the presence of bromine and a yellow precipitate, insoluble In am monium hydroxide shows the presence of iodine.
X + Ag+ → Agx
X represents a halogen – Cl. Br or I.
Question 6.
Hybridization influences the bond length and bond enthalpy in organic compounds: (March – 2011)
a) Compare the bond length and bond strength of C-H bonds formed by sp and sp3 hybridized carbon atoms. Give reason.
b) How many cr and n bonds are present in the following molecules?
i) CH3 – CH2 – CH3
ii) CH3 – C = CH
Answer:
a) sp hybridised carbon is more electronegative than sp3 hybridised carbon. Bond length of C – H bond for sp hybridised carbon is less and that of sp3 hybridised carbon is high.
b) i) 10 σ bonds only
ii) 6 σ bonds and 2n bonds
Question 7.
A series of organic compounds containing a characteristic functional group and represented by a general formula is called a homologous series. (Say – 2011)
a) Classify the following into homologous series and name the series
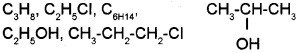
b) Write the general formula of the following homologous series
i) alkaynes
ii) alcohol
iii) chloroalkane
Answer:
a) C3H8, C6H14 – Alakanes
C2H5CI, C4H9CI, CH3CH2CI – Chloroalkanes
C2H5OH, CH3CH(OH)CH3 – Alcohols
b) i) Alkynes – CnH2n+2
ii) Alcohols – CnH2n+2O
iii) Chloroalkanes – CnH2n+2CI
Question 8.
A group of organic compounds, each containing a characteristic functional group forms a homologous series. (March – 2012)
a) Give an example for a homologous series.
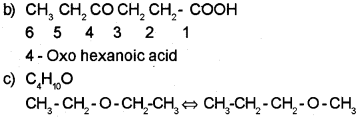
b) Give the IUPAC name of the following compound:
CH3CH2COCH2CH2COOH
c) Write the metamers corresponding to the molecular formula C4H10O.
OR
Different techniques are used for the purification of organic compounds based on their nature.
a) Suggest a suitable method for the separation of a mixture of aniline and water.
b) Give the chemical name of the compound responsible for the blue colour in the Lassaigne’s test for nitrogen.
c) Briefly explain the principle involved in Kjeldahl’s method for the estimation of nitrogen.
Answer:
a) Example for a homologous series.
methane (CH4), ethane (C2H6), Propane (C3H8) etc belong to a homologus series called alkanes.
Question 9.
a) i) Give the complete, condensed and Bond line formula of 2-methyl pentane and chloro cyclo hexane.
ii) Write the IUPAC name of the following compounds.
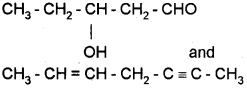
OR
b) i) Give any three types of structural isomers. Give examples.
ii) How will you identify the presence of Halogen by using sodium fusion extract.
iii) Name the method for estimation of Halogen.
Answer:
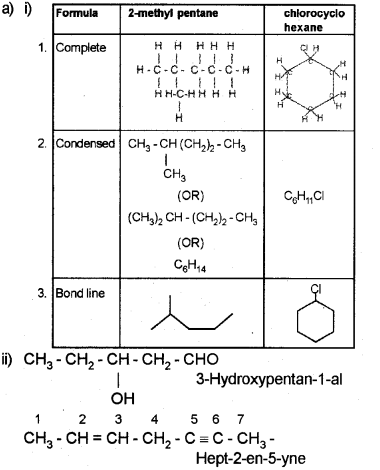
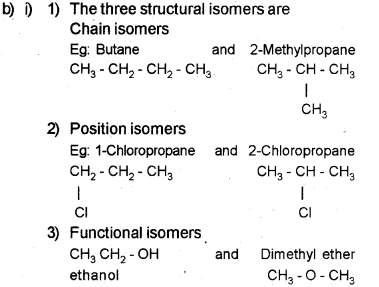
ii) To the sodium fusion extract add dii. HNO3 and AgNO3 solution.
a) White curdy precipitate → Chlorine
b) Pale yellow precipitate → Bromine
C) Yellow precipitate → Iodine
iii) Carius Method
Question 10.
The IUPAC names of alkanes are based on their chain structure. (March – 2013)
a) Give the IUPAC name of
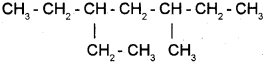
b) Represent 1 – Methyl -3- propyl cyclohexane us-ing bond line notation
c) What is the type of hybridization of C in CH3+? Also predict its shape.
d) Name the type of bond fission resulting in the formation of free radicals
OR
Organic compounds have to be purified before analysis.
a) Which type of liquids can be purified using distillation under reduced pressure? Suggest an example.
b) Name the two main types of chromatographic techniques based on the principle of differential adsorption.
c) In the Lassaigne’s test for halogens, they are precipitated as ……………
d) In what from is nitrogen estimated in the Dumas method?
Answer:
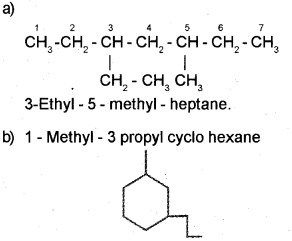
c) Hybridization of ‘C’ in CH+3 is sp2 shape – planer
d) Homolytic bond fission resulting in the formation of free radicals.
OR
a) If a liquid decomposes at its boiling point under atmospheric pressure, it can be purified by distillation under reduced pressure.
Eg : glycerol, H2O2
b) 1) Column chromatography
2) Thin layer chromatography.
c) In the Lassaigne’s test for halogen, they are precipitated as Silver halide
d) In the form of nitrogen gas.
Question 11.
Many chemical properties of organic compounds can be explained on the basis of electron displacement effects. (March – 2013)
a) What is a resonance effect?
b) Categorize the following functional groups into those have +R effct and -R effect:
-NH2, – NO2, – COCH, -OH
a) The movement of electrons in a conjugated system due to the delocalisation of t electron is known as resonance effect.
b) +R effect gps → NH2, – OH
-R effect gps → NO2, – COQH
Question 12.
a) i) Different methods are used to purify organic compounds. Name any three methods of purification. (Say – 2013)
ii) On complete combustion, 0.246g of an organic compound gave 0.198g of CO2 and 0.10149 of H2O. Determine the percentage composition of carbon and hydrogen in the compound.
OR
b) i) What is homologous series?
ii) Hyper conjugation is a general stabilising interaction. Write the hyper-conjugative structures of CH3 – CH2 (ethyl cation).
iii) Write the structures of the following organic compounds.
2, 5, 6- Trimethyloctane.
Hexane -2. 4 – dione.
5- Oxohexanoic acid.
Answer:
a) i) 1) Sublimation
2) Crystallisation
3) Distillation
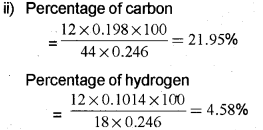
OR
It is a group or series of closely related organic compounds each containing a characteristic functional group, having a general formula, similar chemical properties and a regular gradation in physical properties. The successive members differ from each other in molecular formula by a -CH2 unit.
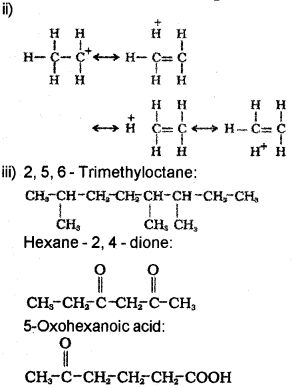
Question 13.
a) Draw the structures of the following compounds. (March – 2014)
2, 3- Dibromo – 1 – phenylpentane
4-Ethyhl – 1 – fluoro -2- nitrobenzene
b) Wide all possible chain isomers of the compound with molecular formula C5H12.
OR
a) Write the complete, condensed and bond line structural formulae of 2-Bromobutane.
b) In the Carius method of estimation of halogen, 0.1 5g of an organic compound gave 0.1 2g ofAgBr. Find the percentage of Br in the compound.
Answer:
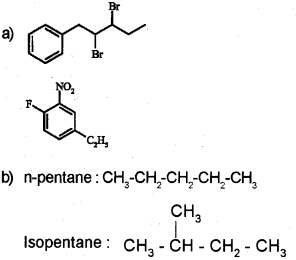
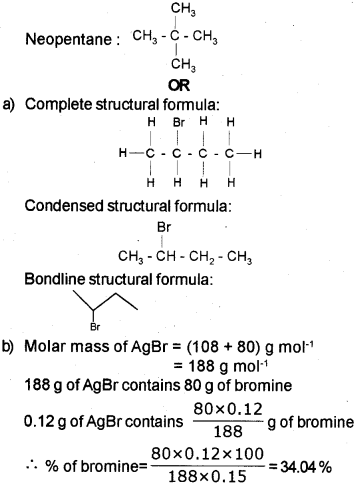
Question 14.
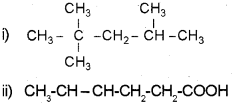
a) Give the IUPAC name of the following compounds.
b) How many’s ‘CT’ and W bonds are present in the following compound?
CH2 = C = CHCH3
c) Write the name of the test used to detect nitrogen, sulphur, halogens and phosphorous present in an organic compound.
d) Explain any one method for the estimation of nitrogen present in an organic compound.
Answer:
a) 0 6-Methyloctan-3-ol
ii) Hex-3-en-1-oicacid
b) 9 sigma bonds and 2 pi bonds.
c) Lassaigne’s test
d) Dumas method or Kjeldahl’s method
Dumas method – The nitrogen-containing organic compound, when heated with copper oxide in an atmosphere of CO2, yields free nitrogen in addition to CO2 and water. Traces of nitrogen oxides formed, if any, are reduced to nitrogen by pass-ing the gaseous mixture over a heated copper gauze. The mixture of gases so produced is collected over an aqueous solution of KOH which absorbs CO2. From the volume of N2 collected the percentage of nitrogen in the organic compound can be determined.
Question 15.
What do you mean by the following terms?
a) Homolytic fission
b) Heterolytic fission (March – 2015)
c) Nucleophiles
d) Electrophiles
OR
Various methods for the purification of organic compounds are based on the nature of the compound and the impurity present in it. Explain the principle involved in the following methods for purification :
a) Distillation
b) Steam distillation
Answer:
a) Homolytic fission – In this one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms. Thus, the movement of a single electron takes place instead of an electron pair. Such cleavage results in the formation of neutral species (atom or group) which contains an unpaired electron. The very reactive species thus formed are free radicals.
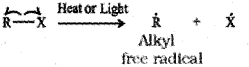
b) Heterolytic fission – In this the bond breaks in such a fasion that the shared pair of electrons remai ns with one of the ragments. After heteroly sis, one atom has a sextet electronic structure and a positive charge and the other, a valence octet with atleast one lone pair and a negative charge. Thus, heterolytic cleavage results in the formation of ions.
![]()
c) Nucleophiles – A reagent that brings an electron pair is called a nucleophile (Nu:). Nucleophile means nucleus seeking. During a polar organic reaction, a nucleophile attacks an electrphilic centre of the substrate which is that specific atom or part of the electrophile that is electron deficient.
e.g. OH’, CN-, R3C:’, H2O:, R3N: etc.
d) Electrophiles-A reagent that takes away an electron pair is called electrophile (E+). Electrophile means electron seeking. During a polar organic reaction, an electrophile attacks at the nucleophilic centre, which is the electron-rich centre of the substrate. Thus, the electrophile receives electron pair from nucleophile when the two undergo bonding interaction.
e.g. CH3 +, CH3CO+, Cl+, NO2+, BF3 etc.
OR
a) Distillation – This is an important method used to separate volatile liquids from non-volatile impurities and the liquids having sufficient difference in their boiling points. Liquids having different boiling points vaporise at different temperatures, On boiling, the vapours of lower boiling component are formed first, which are condensed and the liquid is collected. The vapours of higher boiling component form later and the liquid can be collected separately. For example, chloroform (b.p. 334 K) and aniline (b.p. 457 K) are easily separated by the technique of distillation.
b) seam distillation – This technique is applied to separate substances which are steam volatile and are immiscible with water. In this, steam from a steam generator is passed through a heated flask containing the liquid to be distilled. The liquid boils when the sum of vapour pressures due to the or ganic liquid and that due to water becomes equal to the atmospheric pressure. Thus, the organic liquid vapourises at a lower temperature than its boiling point. The mixture of steam and the volatile or ganic compound is condensed and collected. The compound is later separated from water using a separating funnel. For example, aniline is separated by this technique from aniline – water mixture.
Question 16.
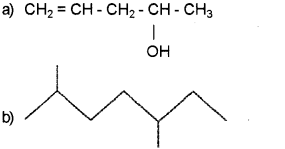
Write the IUPAC names of the following compounds:
Answer:
a) Pent-.4-en-2-oI
b) 2, 5-Dimethylheptane
Question 17.
The bond-line formula ola compound ¡s given below. (Say – 2015)
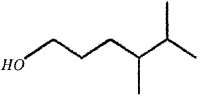
Write its condensed formula and give the IUPAC name.
Answer:
HO(CH2)3 CH(CH3)CH(CH3)2
4, 5DimethyIhexan-1 -ol.
Question 18.
Explain the different types of electron displacement effects in covalent bonds.
(Hint: Inductive effect, reasonance effect, electromeric effect, hyperconjugation)
OR
How is sodium fusion extract prepared? Using this, how will you detect the presence of Nitrogen, Sulphur and Halogen in an organic compound?
Answer:
Inductive Effect: The polarisation of σ – bond caused by the polarisation of adjacent σ – bond is referred to as inductive effect. It is passed on to the subsequent bonds also but the effect decreases rapidly as the number of intervening bonds increases and becomes vanishingly small after three bonds. It is related to the ability of substituents to either withdraw or donate electron density to the attached carbon atoms. Based on this ability, the substituents can be classified as electron-withdrawing (e.g. -NO2) or electron donating groups (e.g. -CH3) relative to hydrogen.
Resonance Effect: It is defined as the polarity produced in the molecule by the interaction of two π – bonds or between a π – bond and lone pair of electrons present on an adjacent atom. The effect is transmitted through the chain. There are two types of resonance effects – Positive resonance effect (The transfer of electrons is away from an atom or substituent group attached to the conjugated system, e.g. -NH2 group in aniline) and negative resonance effect (The transfer of electrons is towards the atom or substituent group attached to the conjugated system, e.g. -NO2 group in nitrobenzene).
Electromeric Effect: It is defined as the complete transfer of a shared pair of π – electrons to one of the atoms joined by a multiple bond on the demand of an attacking reagent. It is a temporary effect. The effect is annulled as soon as the attacking reagent is removed from the domain of the reaction. There are two types of electromeric effects – Positive electromeric effect (The π – electrons of the multiple bond are transferred to that atom to which the reagent gets attached, e.g. H+) and negative electromeric effect (The π – electrons of the multiple bond are transferred to that atom to which the attacking reagent does not get attached, e.g. CN).
Hyperconjugation: It is a general stabilising interaction which involves delocalisation of σ – electrons of C-H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p orbital. The σ – electrons of C-H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p orbital. It is a permanent effect, e.g. Ethyl cation (CH3CH2+).
OR
By fusing a little of the organic compound with metallic sodium. The elements present in the compound are converted from covalent form into the ionic form. The following reactions takes place:
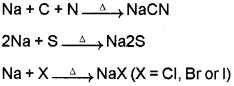
The compounds formed are extracted by boiling it with distilled water.
Test for Nitrogen : The sodium fusion extract is boiled with iron (II) sulphate and then acidified with concentrated sulphuric acid. The formaation of Prussian blue colour confirms the presence of nitrogen.
Test for Sulphur : The sodium fusion extract is acidified with acetic acid and lead acetate is added to it. A black precipitate of lead sulphide indicates the presence of sulphur.
Test for Halogen : The sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. A white precipitate, soluble in ammonium hydroxide shows the presence of chlorine, a yellowish precipitate, sparingly soluble in ammonium hydroxide shows the presence of bromine and a yellow precipitate, insoluble in ammonium hydroxide shows the presence of iodine.
Question 19.
a) Give the IUPAC name of the following compounds. (March – 2016)
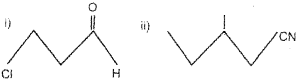
b) Phenol exhibit resonance.
i) Draw the resonance structures of phenol.
ii) Predict the directive influence of-OH group in Benzene ring.
OR
a) Write the structural formula of the following compounds.
i) Pent-4-en-2-ol
ii) 6-Hydroxy heptanal
b) Reagents which attack organic compounds may be classified as electrophiles, nucleophiles and free radicals.
i) Explain nucleophiles and electrophiles with suitable examples.
ii) Name the type of the fission of a covalent bond which gives free radicals.
crystallises out and is removed by filtration. Repeated crystallisation becomes necessary for the purification of compounds containing impurities of comparable solubilities.
OR
a) i) 3-Bromo-3-chloroheptane
ii) 4-Ethyl-2-methylaniline
b) (CH3)3C+ is more stable than CH3CH2+ since the stability of carbocations decreases in the order 3° > 2° > 1° due to electron releasing inductive effect (+l effect) of alkyl group and hyperconjugation. Greater the number of alkyl groups on the carbon carrying +ve charge greater would be the dispersal of positive charge and hence more would be the stability of the carbocation. Also, greater the number of alkyl groups attached to a positively charged carbon atom, greater is the dispersal of positive charge through hyperconjugation (overlap of the empty p-orbital on carbon with the sp3-s sigma orbital of the adjacent carbon atom) and hence greater is the stability of carbocation.
c) This method is used to purify liquids having very high boiling points and those, which decompose at or below their boiling points. Such liquids are made to boil at a temperature lower than their normal boiling points by reducing the pressure on their surface with the help of a water pump or vacuum pump.
We hope the Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 12 Organic Chemistry Some Basic Principles and Techniques help you. If you have any query regarding Kerala Plus One Chemistry Chapter 12 Organic Chemistry Some Basic Principles and Techniques, drop a comment below and we will get back to you at the earliest.
