Plus One Chemistry Chapter Wise Previous Questions Chapter 1 Some Basic Concepts of Chemistry is part of Kerala Plus One Chemistry Chapter Wise Previous Year Questions and Answer. Here we have given Plus One Chemistry Previous Questions Chapter 1 Some Basic Concepts of Chemistry.
Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 1 Some Basic Concepts of Chemistry
Question 1.
Calculate the number of moles of 02 required to produce 240g of MgO by burning Mg metal. (Atomic mass: Mg = 24, 0 = 16) (March – 2019)
Answer:
Mg + 1/2 O2 → MgO
Molecular mass of MgO = 24 + 16 = 40
No. of moles of MgO present in 240g of MgO = 6 moles
As per the balanced equation, we have to use 6 moles of Mg and 3 moles of O2 in order to get 240g of MgO.
Question 2.
If the mass percent of the various elements of a compound is known, its empirical formula can be calculated. (March – 2010)
a) What is mass percent? Give its mathematical expression.
b) A compound contains 4.07% hydrogen, 24.27% carbon and 71.65% chlorine. Its molecular mass is 98.96. What are the empirical and molecular formulae?
Answer:
a) Mass percentage of a component in a solution is the weight of that component present in 100gm of the solution.
![]()
b)
| Element | % | Relative no. of atoms | Dividing by smallest factor | Rate of no. atoms |
| C | 24.27% | 24.2712=2.02 | 2.012.01=2.01 | 1 |
| H | 4.07% | 4.071=4.07 | 4.072.01=2.02 | 2 |
| Cl | 71.65% | 71.6535.5=2.018 | 2.012.01=1 | 1 |
Empirical formula = CH2CI
Molecular mass = 98.96
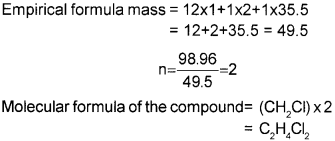
Question 3.
One mole is the amount of substance that contains as many elementary particles as 12g of 12C isotope of carbon. (Say – 2010)
a) What do you mean by molar mass of a compound?
b) Calculate the number of moles in 1 litre of water (Density of water is 1g/mL). Also calculate the number of water molecules in 1 litre of water.
Answer:
a) The mass of one mole of any substance is called the molar mass.
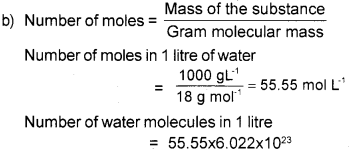
Question 4.
The laws of chemical combination are the basis of the atomic theory. (March – 2011)
a) Name the law of chemical combination illustrated by the pair of compounds, CO and CO2.
b) State and explain the law of conservation of mass.
c) Calculate the molarity of a solution containing 8g of NaOH in 500 mLofwater.
Answer:
a) Law of multiple proportions.
b) Matter can neither be created nor be destroyed.
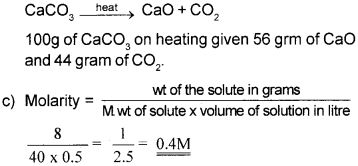
Question 5.
The laws of chemical combination govern the formation of compounds from elements. (Say – 2011)
a) State the law of conservation of mass. Who put forward this law?
b) The following data are obtained when dinitrogen and dioxygen react together to from different compounds.
| SI. No. | Mass of dinitrogen (g) | Mass of dioxygen (g) |
| 1 | 14 | 16 |
| 2 | 14 | 32 |
| 3 | 28 | 48 |
| 4 | 28 | 80 |
Which law of chemical combination is illustrated by the above experimental data? Explain.
Answer:
a) It states that matter can neither be created nor destroyed.
OR
The total mass of the reactants in a chemical reaction is equal to the total mass of the products formed. This law was proposed by Antoine Lavoisier,
b) Law of multiple proportions proposed by John Dalton.
According to this law if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
Here, the oxides of nitrogen are,
![]()
The different masses of oxygen which combine with a fixed mass (28g) of nitrogen are in the ratio 32:64:48:80 = 2:4:3:5, which is a simple whole-number ratio. Hence, the law is verified.
Question 6.
The combination of elements ot form compounds is governed by the laws of chemical combination. (March – 2012)
a) Hydrogen combines with oxygen to form compounds, namely water and hydrogen peroxide. State and illustrate the related law of chemical
combination.
b) What is meant by ‘limiting reagent’ in a chemical reaction?
c) 28 g of nitrogen is mixed with 12 g of hydrogen to form ammonia as per the reaction, N2 + 3H2 → 2NH3. Which is the ‘limiting reagent’ in this reaction. [Atomic masses : N = 14, H = 1]
Answer:
a) Law of multiple proportions

Here, the masses of oxygen (ie, 16g and 32g)which combine with a fixed mass of hydorgen (2g) bear a simple ratio ie 16:32 or 1:2.
Law of multiple proportions states that if two elements combine to form two or more compound, the weights of one of elements which combine with a fixed weight of the other in these compound. bear simple whole-number ratio by weight.
b) The reactant which is completely used up first in a reaction is called the limiting reactant.
c) N2 – 3H2 → 2NH3
28g 6g → 34g
28g 12g → (given data)
∴ N2 is the limiting reagent. (N2 is completely used up in a reaction)
Question 7.
a) Mole is a very large number to indicate the number of atoms, molecules, etc. Write another name for one mole. (Say – 2012)
b) i) How the molecular formula is different from that of the Empirical formula?
ii) An organic compound on analysis gave the following composition.
Carbon=40%, Hydrogen=6.66% and oxy- gen=53.34%. Calculate its molecular formula if its molecular mass is 90.
Answer:
a) 1 mole = gram atomic mass or 1 gram atom
1 mole = gram molecular mass or 1 gram molecule
b) 1) Empirical formula of a compound is defined as the simplest formula that gives the ratio of the various elements in a molecules.
Eg: Empirical formula of benzene is CH. Molecular formula of a compound gives actual number of atoms of each element present in a molecular of the compound, eg: molecular formula of benzene is C6H6.
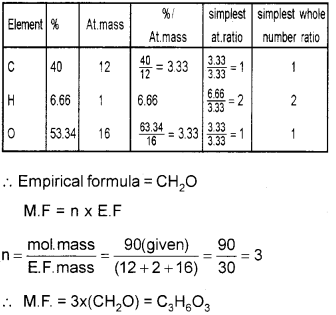
Question 8.
The mole concept helps in handling a large number of atoms and molecules in stoichiometric calculations. (March – 2013)
a) Define 1 mol.
b) What is the number of hydrogen atoms in 1 mole of methane (CH4)?
c) Calculate the amount of carbon dioxide formed by the complete combustion of 80g of methane as per the reaction :
CH4(g) + 202(g) → CO2(g) + 2H2O(g)
(Atomic masses: C = 12.01 u.
H = 1.008u,
O = 16u)
Answer:
a) I mole is defined as the amount of any substance which contains Avogadro number of particles (ie atoms, ions or molecules)
b) I mole methane contain 4 Flydrogen atoms (ie n = 4)
No. of hydrogen atoms
= No. of mole x NA x n
= 1 x 6.023 x 1023 x 4
= 24.092 x 1023 atoms
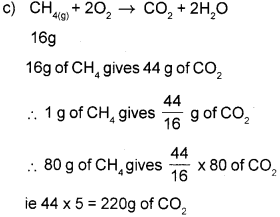
Question 9.
a) Atoms have very very small mass and so usually the masses of atoms are given relative to a standard called atomic mass unit. What is the Atomic Mass Unit (AMU)? (Say – 2013)
b) In a reaction A + B2 → AB2, identify the limiting reagent in the reaction mixture containing 5 mol A and 2.5 mol B.
c) Calculate the mass of NaOH required to make 500 mL of 0.5 M aqueous solution (Molecular mass of NaOH = 40).
Answer:
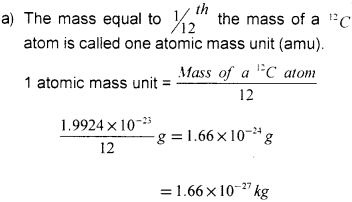
b) As per the reaction 1 mol of A reacts completely with 1 mole of B2 to form 1 mole of AB2. Thus, 5 mole of A requires 5 mole of B2. Flence, B2 is the limiting reagent.
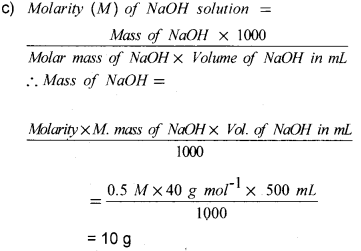
Question 10.
a) How many moles of dioxygen are present in 64 g of dioxygen? (Molecular mass of dioxygen is 32).
b) The following data were obtained when dinitrogen (N2) and dioxygen (O2) react together to form different compounds.
| Mass of N2 | Mass of 02 |
| 14 g | 16g |
| 14 g | 32 g |
| 28 g | 32 g |
| 28 g | 80 g |
Name the law of chemical combination obeyed by the above experimental data.
c) Define empirical formula. How is it related to the molecular formula of a compound?
Answer:
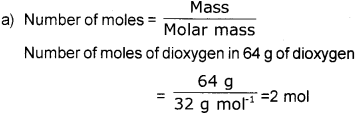
b) Law of multiple proportions.
c) Empirical formula is the simplest formula which represents the simplest whole number ratio of various atoms present in a compound.
Molecular formula = n x (Empirical formula) where ‘n’ = 1, 2, 3, ….
Question 11.
Hydrogen combines with oxygen to form two different compounds, namely, water (H2O) and hydrogen peroxide (H2O2) (August – 2014)
a) Which law is obeyed by this combination?
b) State the law
c) How may significant figures are present in the following?
i) 0.0025
ii) 285
Answer:
a) Law of Multiple Proportions
b) It states that if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
c) i) 0.0025 – 2 significant figures
ii) 285 – 3 significant figures
Question 12.
‘A given compound always contains exactly the same proportion of elements by weight’. (March – 2015)
a) i) Name the above law.
ii) Write the name of the scientist who proposed this law.
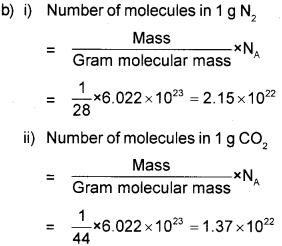
b) Calculate the number of molecules in each of the following:
i) 1g N2
ii) 1g CO2
(Given that Na is 6.02 x 1023, molecular mass of N2 is 28 and CO2 is 44).
Answer:
a) i) Law of Definite Proportions Or Law of Definite Composition
ii) Joseph Proust
Question 13.
12g of 12C contains Avogadro’s number of carbon atms. (Say – 2015)
a) Give the Avagadro’s number.
b) The mass of 2 moles of ammonia gas is
i) 2g
ii) 1.2 x 1022g
iii) 17g
iv) 34g
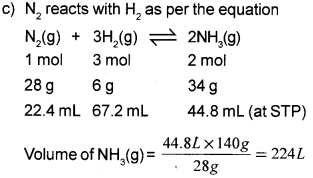
c) Calculate the volume of ammonia gas produced at STP when 140g of nitrogen gas reacts with 30g of hydrogen gas. (Atomic masses : N = 14u, H = 1u)
Answer:
a) 6.022 x 1023
b) iv) 34 g
Question 14.
a) When nitrogen and hydrogen combines to form ammonia, the ratio between the volumes of gaseous reactants and products is 1:3:2. Name the law of chemical combination illustrated here. (March – 2016)
b) A compound is made up of two elements A and B, hasA= 70%, B = 30%. The relative number of moles of A and B in the compound are 1.25 and 1.88 respectively. If the molecular mass of the compound is 160, find the molecular formula of the compound.
Answer:
a) Gay Lussac’s law of gaseous volumes
b) The empiricial formula of the compound is A2B3
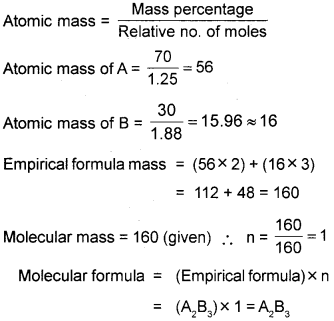
Question 15.
The empirical formula represents the simplest whole number ratio of various atoms present in a compound. (Say – 2016)
a) Give the relationship between empirical formula and molecular formula.
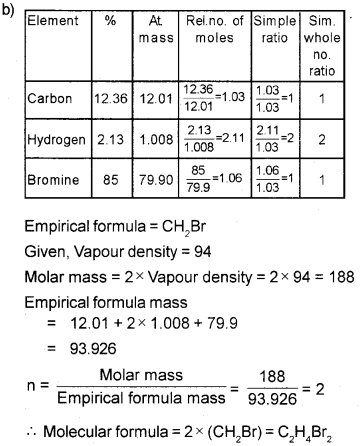
b) An organic compound has the following percentage composition C = 12.36%, H = 2.13%, Br = 85%. Its vapour density is 94. Find its molecular formula.
c) What is mole fraction?
Answer:
a) Molecular formula is a whole number multiple of the empirical formula.
i.e., Molecular formula = nx empirical formula where, n = 1, 2, 3…
![]()
c) Mole fraction of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all the components in solution.
If a substance ‘A’ dissolves in substance ‘B’ and their number of moles are nA and nB respectively; then the mole fractions of A and B are given as Mole fraction of A.
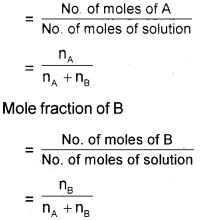
Question 16.
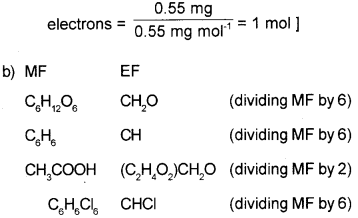
a) Determine the number of moles present in 0.55mg of electrons. (March – 2017)
i) 1 mole
ii) 2 moles
iii) 1.5 moles
iv) 0.5 mole
b) Give the empirical formula of the following.
C6H12O6, C6H6, CH3COOH, C6H6Cl6
c) Two elements, carbon and hydrogen combine to form C2H6, C2H4 and C2H2. Identify the law illustrated here.
Answer:
a) i) 1 mol
[Explanation: Mass of 1 electron
= 9.1094 x 10-31 kg
= 9.1094 x 10-25 mg
Mass of 1 mole of electrons
= 6.022 x 1023 X 9.1094 x 10-25 mg
= 0.55 mg
∴ Number of moles present in 0.55 mg of
c) Law of multiple propotions [Explanation:
In C6H6:24g Carbon + 6g Hydrogen
In C2H4:24g Carbon + 4g Hydrogen
In C2H2:24g Carbon + 2g Hydrogen
Here, the masses of hydrogen (6g, 4g and 2g)
which combine with a fixed mass of carbon (24g)
bear a simple ratio, i.e., 6 : 4 : 2 = 3 : 2 : 1]
We hope the Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 1 Some Basic Concepts of Chemistry help you. If you have any query regarding Kerala Plus One Chemistry Chapter Wise Previous Questions Chapter 1 Some Basic Concepts of Chemistry, drop a comment below and we will get back to you at the earliest.
