Kerala Plus One Botany Notes Chapter 10 Respiration in Plants
How are respiration and photosynthesis-related?
Green plants and cyanobacteria can prepare their own food by the process of photosynthesis, they trap light energy and convert it into chemical energy that is stored in the bonds of carbohydrates (Macromolecules) like glucose, sucrose, and starch.
By Cellular respiration, food materials undergo breakdown that release energy and the trapping of this energy for the synthesis of ATP.
ATP is called the energy currency of the cell why?
ATP is broken down whenever (and wherever) energy needs to be utilised. Hence, ATP acts as the energy currency of the cell.
Seat of photosynthesis and respiration
Photosynthesis takes place within the chloroplasts whereas the breakdown of complex molecules to yield energy takes place in the cytoplasm and in the mitochondria (also only in eukaryotes).
The compounds that are oxidized during respiration are known as respiratory substrates.
Eg. Proteins, fats, and even organic acids.
Do Plants Breathe?
Plants require O2 for respiration and give out CO2 and H2O as end products and release energy most of which is given out as heat.
Respiration is least important to plants than animals
Roots, stems, and leaves respire at rates far lower than animals When cells photosynthesize O2 is released within the cell.
But some cells live where oxygen may or may not be available.
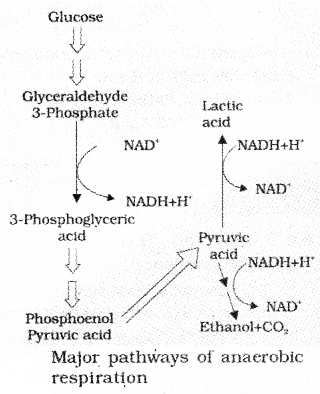
All living organisms retain the enzymatic machinery to partially oxidise glucose without the help of oxygen. This breakdown of glucose to pyruvic acid is called glycolysis.
Glycolysis
The term glycolysis -(Greek words, glycols for sugar, and lysis for splitting).
The scheme of glycolysis was given by Gustav Embden, Otto Meyerhof, and J. Parnas, Hence glycolysis is called an EMP pathway.
In anaerobic organisms, it is the only process in respiration.
Glycolysis occurs in the cytoplasm of the cell and is present in all living organisms.
In this process, Glucose undergoes partial oxidation to form two molecules of pyruvic acid.
Steps lead to end products of glycolysis

1. Glucose and fructose are phosphorylated to give rise to glucose-6-phosphate by the activity of the enzyme hexokinase.
2. This phosphorylated form of glucose then isomerises to produce fructose-6-phosphate.
3. In this pathway, ATP is utilised at two steps: first in the conversion of glucose into glucose 6-phosphate and second in the conversion of fructose 6-phosphate to fructose 1,6 diphosphate).
4. The fructose 1,6-diphosphate is split into dihydroxyacetone phosphate and 3 phosphoglyceraldehydes (PGAL). In this step NADH +H+ is formed from NAD+.
5. 3-phosphoglyceraldehyde (PGAL) is converted to 1,3 bisphosphoglycerate (DPGA).
6. The conversion of DPGA to 3-phosphoglyceric acid (PGA), is also an energy-yielding process; this energy is trapped by the formation of ATP.
7. 3-phosphoglyceric acid (PGA) is converted into 2 phosphoglycerates.
8. 2 phosphoglycerates are converted into 2 phosphoenol pyruvic acid. ATP is synthesized during the conversion of PEP to pyruvic acid.
9. 2 phosphoenol pyruvic acid undergoes dephosphorylation to form 2 molecule of pyruvic acid
The fate of pyruvic acid
It involves
- Lactic acid fermentation
- Alcoholic fermentation
- Aaerobic respiration.
Fermentation takes place under anaerobic conditions in many prokaryotes and unicellular eukaryotes.
The complete oxidation of glucose to CO2 and H2O occurs in organisms that adopt Krebs’ cycle which is also called as aerobic respiration. This requires an O2 supply.
Fermentation
In fermentation, glucose undergoes incomplete oxidation and forms CO2 and ethanol
The enzymes, pyruvic acid decarboxylase, and alcohol dehydrogenase catalyze these reactions.
Fermentation occurs in the presence of yeast
Yeasts poison themselves to death when the concentration of alcohol reaches about 13 percent. Some organisms like bacteria produce lactic acid from pyruvic acid.
The lactic acid in eukaryotic cell
In animal muscle cells during exercise, when oxygen is inadequate for cellular respiration, pyruvic acid is reduced to lactic acid by lactate dehydrogenase.
The reducing agent is NADH+H* which is re oxidised to NAD+ in both the processes.
In both lactic acid and alcohol fermentation, less than seven percent of the energy in glucose is released.
In eukaryotes second step after glycolysis take place within the mitochondria and this requires O2.
It is aerobic respiration leads to complete oxidation of carbohydrate in the presence of oxygen and releases CO2, water and a large amount of energy.
This type of respiration is most common in higher organisms.
Aerobic Respiration
The second step of Aerobic respiration takes place within the mitochondria.
The product of glycolysis- pyruvate is transported from the cytoplasm into the mitochondria.
First step of oxidation of pyruvic acid
In the mitochondrial matrix, pyruvate undergoes oxidative decarboxylation by pyruvic dehydrogenase. The reactions require the participation of several coenzymes, including NAD+ and Coenzyme A.

During this process, two molecules of NADH are produced from the metabolism of two molecules of pyruvic acid.
The acetyl CoA then enters a cyclic pathway, tricarboxylic acid cycle(Krebs’ cycle) after the scientist Hans Krebs who first elucidated it.
Tricarboxylic Acid Cycle
The TCA cycle starts with the condensation of acetyl group with oxaloacetic acid (OAA) and water to yield citric acid.
The reaction is catalysed by the enzyme citrate synthase and a molecule of CoA is released.
It is followed by two successive steps of decarboxylation, leading to the formation of alpha-ketoglutaric acid and then succinyl-CoA. In the remaining steps, Succinic acid is oxidised to OAA.

Which step of the Krebs cycle substrate-level phosphorylation occurs?
During the conversion of succinyl-CoA to succinic acid, a molecule of GTP is synthesised. This is substrate-level phosphorylation.
At three sites in the cycle where NAD+ is reduced to NADH + H+ and one site where FAD+ is reduced to FADH2.

In the mitochondrial matrix, pyruvate is broken down to release.
8 molecules of NADH + H+
2 molecules of FADH2
2 molecules of GTP and
3 molecules of CO2
Electron Transport System (ETS) and Oxidative Phosphorylation

The metabolic pathway through which the electron passes from one carrier to another is called the electron transport system (ETS).
It is present in the inner mitochondrial membrane.
Reduced coenzyme like NADH(complex) in the mitochondrial matrix is oxidised and release 2 electrons and 2protons
Electrons and protons are transferred to FMN, it reduced to FMNH2
It breaks and releases protons and electrons .protons go to intermembrane space but electrons reach ubiquinone.
Ubiquinone also receives reducing equivalents via FADH2 (complex II).
The reduced ubiquinone is then oxidised with the transfer of electrons to cytochrome c via cytochrome bc1 complex (complex III).
Electron Transport System (ETS)
Cytochrome c acts as a mobile carrier for the transfer of electrons between complex III and IV.
Complex IV refers to cytochrome c oxidase complex containing cytochromes a and a3.
Oxidation of one molecule of NADH gives rise to 3 molecules of ATP, while that of one molecule of FADH2 produces 2 molecules of ATP.
Oxygen acts as the final hydrogen acceptor.
Oxidative phosphorylation in mitochondria
In ETS the energy of oxidation-reduction is utilised for the production of proton gradient required for phosphorylation. This process is called oxidative phosphorylation.
Chemiosmosis (proposed by peter Mitchel)
The energy released during the electron transport system is utilised in synthesizing ATP with the help of ATP synthase (complex V) called chemiosmosis.
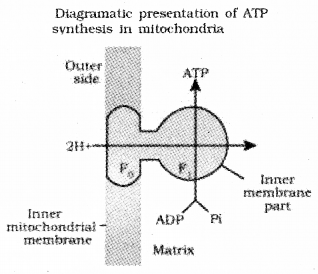
F1 – F0/exosomes
This complex consists of two major components, F1 and Fo.
The F1 headpiece is a site for synthesis of ATP from ADP and inorganic phosphate.
F0 is an integral membrane protein complex act as a channel through which protons cross the inner membrane.
For each ATP produced, 2H+ passes through F0 from the intermembrane space to the matrix down the electrochemical proton gradient.
The Respiratory Balance Sheet
How many ATP molecules are produced in Aerobic respiration?
In aerobic respiration, the number of ATP molecules produced or utilized in glycolysis, TCA cycle and ETS gives the net gain of 36 ATP molecules
Fermentation accounts for only a partial breakdown of glucose whereas in aerobic respiration it is completely degraded to CO2 and H2O.
How many ATP molecules are produced in Fermentation?
In fermentation there is a net gain of only two molecules of ATP for each molecule of glucose degraded NADH is oxidized to NAD+ rather slowly in fermentation.
Amphibolic Pathway
It involves two processes anabolism and catabolism.
For example, fats is broken down into glycerol and fatty acids. Then fatty acids degraded to acetyl CoA and enter the pathway.
Glycerol enters the pathway after being converted to PGAL.
The proteins are degraded by proteases and the individual amino acids enter the pathway at some stage within the Krebs’cycle as pyruvate or acetyl CoA.
Is it true both catabolism and anabolism occur in fat metabolism?
Fatty acids( substrate) are broken down to acetyl CoA before entering the respiratory pathway. But when the organism needs to synthesize fatty acids, acetyl CoA withdrawn from the respiratory pathway for it.
Hence, the respiratory pathway involves the breakdown and synthesis of fatty acids, i.e catabolism, and anabolism respectively. Hence it is considered as an amphibolic pathway.

Respiratory Quotient
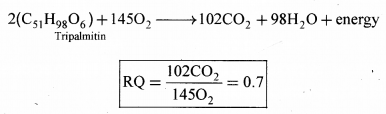
Definition: The ratio of the volume of CO2 evolved to the volume of O2 consumed in respiration is called the respiratory quotient (RQ) or respiratory ratio.

Respiratory quotient of some respiratory substrates
1. Carbohydrates: When carbohydrates are completely oxidised, the RQ is 1, because equal amounts of CO2 and O2 are evolved and consumed, respectively
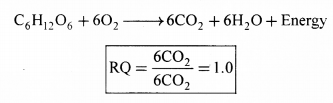
2. Fats: If fats are used in respiration, the RQ is less than 1.
3. Proteins: When proteins are respiratory substrates the ratio is 0.9.
4. Organic acids: When organic acids are respiratory substrates, the ratio is more than one.
