Physical and Chemical Properties of Group 17 Elements
Group 17 Elements: The Halogens
- The elements in Group 17 are:
Fluorine Chlorine Bromine Iodine Astatine - These elements are known as halogens.
- The elements in Group 17 are:
- (a) Halogen is a Greek word which means salt-former’.
(b) This is because- halogens are reactive non-metals.
- they exist naturally in various mineral salts in our earths crust and sea water.
Molecur formulae of halogens
- Halogens exist as diatomic covalent molecules.
- Table shows the molecular formulae of halogens.
Halogen Fluorine Chlorine Bromine Iodine Astatine Molecular formula F2 Cl2 Br2 I2 At2
Electronegativity of halogens
- Definition:
- The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus.
- Halogens are very electronegative.
- Table shows the electronegativities of halogens.
Element Fluorine Chlorine Bromine Iodine Electronegativity (Pauling scale) 4.0 3.0 2.8 2.5 - (a) The electronegativity of halogens decreases when going down the group.
(b) This can be explained as below:- The number of shells occupied with electrons in the atoms of halogens increases when going down the group.
- This causes the outermost occupied shell to become further away from the nucleus and is screened by more inner shells containing electrons.
- Hence, the strength of the nucleus to attract electrons becomes weaker.
People also ask
- What is the periodic table of the elements?
- Physical and Chemical Properties of Group 1 Elements
- Physical and Chemical Properties of Group 18 Elements
- What do you mean by transition metals?
- What is the Need for Classification of Elements?
- Modern Periodic Table and Its Significance
- How did Mendeleev Arrange the Periodic Table?
- Periodic Trends in Properties of Elements
- What is Valency and Atomic Radius?
- What are Metallic and Nonmetallic Properties?
Physical properties of Group 17 elements
1. Table shows some physical properties of Group 17 elements.
| Element | Fluorine | Chlorine | Bromine | Iodine |
| Molecular formula | F2 | Cl2 | Br2 | I2 |
| Proton number | 9 | 17 | 35 | 53 |
| Atomic radius (nm) | 0.071 | 0.099 | 0.114 | 0.133 |
| Density (g cm-1) | 0.0017 | 0.0032 | 3.13 | 4.94 |
| Melting point (°C) | -220 | -101 | -7 | 114 |
| Boiling point (°C) | -188 | -35 | 59 | 184 |
2. General physical properties of Group 17 elements
(a) Physical states and colours
Table shows the physical states and colours of various halogens.
| Halogen | Physical state and colour |
| Fluorine | Pale yellow gas |
| Chlorine | Greenish-yellow gas |
| Bromine | Reddish-brown liquid |
| Iodine | Purplish-black solid |
The colours of the halogens become darker when going down Group 17.
(b) All halogens have low melting and boiling points.
This can be explained as below:
- The halogen molecules are held together by weak van der Waals forces of attraction.
- Hence, small amount of heat energy is needed to overcome it during melting or boiling.
All halogens have low densities.
All halogens do not conduct electricity.
All halogens are weak conductors of heat.
3. Trend of change in the physical properties
However, some of the physical properties mentioned above vary gradually when going down Group 17, as shown in Table.
| Group 17 elements | Trend of change in the physical properties | ||
 | The atomic radius (atomic size) of the halogens increases gradually down the group. Reason: | Although halogens have low melting and boiling points, the melting and boiling points increase down the group. Reason: | Although halogens have low densities, the density of the halogens increases gradually down the group. |
Chemical properties of Group 17 elements
1. Table shows the electron arrangements of halogens.
| Element | Electron arrangement |
| Fluorine | 2.7 |
| Chlorine | 2.8.7 |
| Bromine | 2.8.18.7 |
| Iodine | 2.8.18.18.7 |
| Astatine | 2.8.18.32.18.7 |
2. Similar chemical properties
- All halogens exhibit similar chemical properties.
- This is because all the atoms of halogens have 7 valence electrons.
3. Reactivity
- Although halogens exhibit similar chemical properties, they differ in reactivity.
- The reactivity of halogens decreases when going down Group 17.

- The reactivity of a halogen is measured by how easily its atom accepts one electron to achieve a stable noble gas electron arrangement (octet electron arrangement).
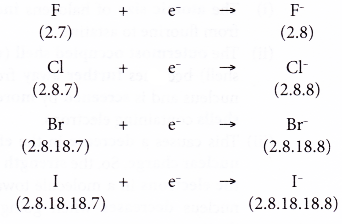
- The easier the atom of a halogen gains one electron, the more reactive is the halogen,
Explanation:
The decrease in the reactivity down Group 17 can be explained as follows.
- All halogens have seven valence electrons.
- Each halogen atom will gain one electron to achieve a stable octet electron arrangement. Hence, an ion with a charge of -1 is formed.
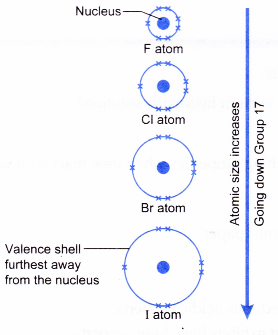
- When going down Group 17, the atomic size of halogens increases.
- The outermost occupied shell becomes further away from the nucleus and is screened by more inner shells containing electrons.
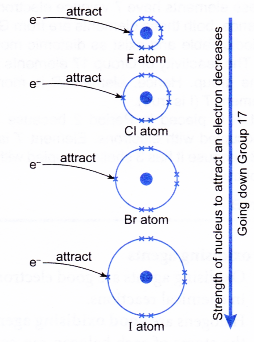
- This means that the effective nuclear charge exerted on the outer valence shell decreases when going down the group.
- Hence, the attractive forces exerted by the nucleus to attract one more electron into the valence shell decreases when going down the group.
- This causes the reactivity of halogens to decrease down the group.
4. As oxidising agents
- Oxidising agents are good electron acceptors in chemical reactions.
- Halogens are good oxidising agents because the atoms of each halogen can easily accept one electron to achieve a stable octet electron arrangement.
- The strength of the halogens as oxidising agents decreases when going down Group 17.

This is because the strength of the nucleus of a halogen atom to attract one more electron into the valence shell (outermost shell) decreases when going down the group.
5. Electronegativity
Halogens are very electronegative.
However, the electronegativity of the halogens decreases when going down Group 17.

This can be explained as below:
- The atomic size of halogens increases from fluorine to astatine.
- The outermost occupied shell (valence shell) becomes further away from the nucleus and is screened by more inner shells containing electrons.
- This causes a decrease in the effective nuclear charge. So, the strength to pull the electrons in a molecule towards its nucleus decreases when going down the group.
- Hence, the electronegativity decreases from fluorine to astatine
6. Group 17 elements exhibit similar chemical properties in their reactions with
(a) water to produce two types of acids.
(b) iron to produce iron(III) halides.
(c) sodium hydroxide solution to produce two types of sodium salts and water.
7. To predict the properties of astatine
- Astatine is placed below iodine in Group 17 of the Periodic Table.
- Hence, astatine is expected to react with water, iron and sodium hydroxide solution in the similar way as iodine but these reactions are slower (less reactive) than iodine.
- For example:
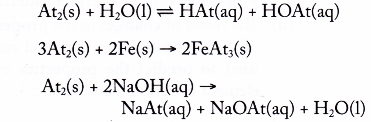
8. Safety precautions in handling Group 17 elements
- Fluorine is not only poisonous but also a very dangerous reactive gas, whereas astatine is radioactive. Therefore, these two elements are not used in school laboratories.
- Chlorine gas, bromine gas and iodine vapour are poisonous.
- Iodine vapour is harmful to the respiratory system of living things including human beings.
- Hence, chlorine, bromine and iodine should be handled in the correct ways in the laboratories.
- The following safety precautions must be taken when handling these halogens.
(i) Handle the halogens in a fume chamber.
(ii) Wear safety goggles and gloves.
Chemical Properties of Group 17 Elements Experiment
Aim: To investigate the chemical properties of Group 17 elements.
Problem statement: How do halogens react with water, iron and sodium hydroxide solution?
A. Reactions of halogens with water
Hypothesis: Halogens form acidic solutions and also show bleaching properties when they react with water.
Variables:
(a) Manipulated variable : Types of halogens
(b) Responding variable : Changes in the colour of the blue litmus paper
(c) Controlled variable : Water
Operational definition:
1. When the blue litmus paper turns red, the solution formed exhibits acidic property.
2. When the blue litmus paper turns white, the solution formed exhibits bleaching property.
Materials: Chlorine gas (produced by mixing potassium manganate(VII) crystals with concentrated hydrochloric acid), liquid bromine, solid iodine, distilled water and blue litmus paper.
Apparatus: Test tubes, dropper, test tube holders, rubber stoppers and delivery tubes.
Procedure:
Safety measures
- Chlorine gas, liquid bromine and solid iodine are poisonous.
- Wear gloves and safety goggles when handling these halogens.
- Carry out the experiment in a fume chamber.
I. Chlorine with water
- A few pieces of potassium manganate(VII) crystals are placed in a test tube.
- Concentrated hydrochloric acid is added just enough to cover the potassium manganate(VII) crystals.
- The liberated chlorine gas is then passed through 5 cm3 of distilled water in another test tube, as shown in Figure.
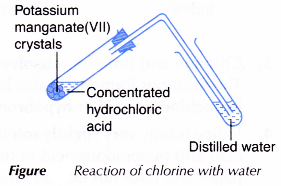
- The colour of the solution formed is recorded.
- The solution formed is tested with a piece of blue litmus paper.
- All the changes are recorded.
II. Bromine with water
- Two drops of liquid bromine are added into a test tube containing 5cm3 water and shaken well, as shown in Figure.
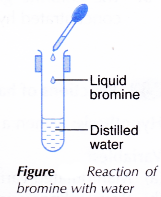
- The colour of the solution formed is recorded.
- The solution formed is tested with a piece of blue litmus paper.
- All the changes are recorded.
III. Iodine with water
- A small piece of solid iodine is added into a test tube containing 5 cm3 of distilled water.
- The test tube is closed with a rubber stopper and shaken strongly, as shown in Figure.
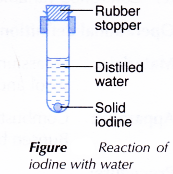
- The colour of the solution formed is recorded.
- The solution formed is tested with a piece of blue litmus paper.
- All the changes are recorded.
Observations:
| Halogen | Observation |
| Chlorine | The greenish-yellow gas dissolves rapidly in water to produce a pale yellow solution. This solution turns blue litmus paper red and then white. |
| Bromine | The reddish-brown liquid dissolves slowly in water to form a yellowish-brown solution. This solution turns blue litmus paper red and then white. |
| Iodine | Only a very small amount of the purplish-black crystal dissolves very slowly in water to produce a pale yellow solution. This solution has no effect on blue litmus paper. |
Discussion:
- The solubility of halogens in water decreases when going down Group 17.
- Halogens react with water to produce solutions.
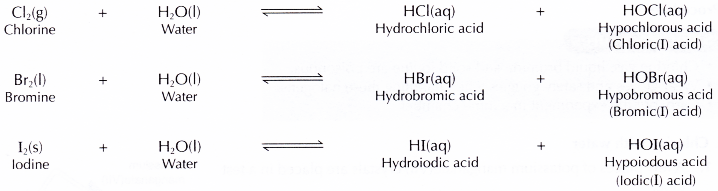
- Chlorine and bromine dissolve readily in water forming acidic solutions which turn blue litmus paper red. The solutions formed are also bleaching agents which then turn the litmus paper white due to the presence of hypochlorous acid or hypobromous acid.
- Iodine is only very slightly soluble in water. Only very little iodine dissolves in water. The amount of hydroiodic acid and hypoiodous acid in the iodine water is so little that it is unable to change the colour of blue litmus paper and does not exhibit bleaching properties.
- The chlorine gas used in this experiment is prepared by mixing potassium manganate(VII) crystals with concentrated hydrochloric acid. The chemical equation for this reaction is:
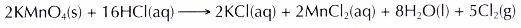
B. Reactions of halogens with iron
Hypothesis: When a halogen reacts with iron, an iron(III) halide is formed.
Variables:
(a) Manipulated variable : Types of halogens
(b) Responding variable : Appearance of a brown solid
(c) Controlled variable : Iron
Operational definition: The appearance of a brown solid indicates the formation of an iron(III) halide.
Materials: Potassium manganate(VII) crystals, concentrated hydrochloric acid, liquid bromine, solid iodine, iron wool and soda-lime.
Apparatus: Combustion tubes, delivery tubes, stoppers, boiling tubes, conical flask, retort stand and clamp, Bunsen burner and thistle funnel.
Procedure:
I. Chlorine with iron
- The arrangement of apparatus as shown in Figure is set up.
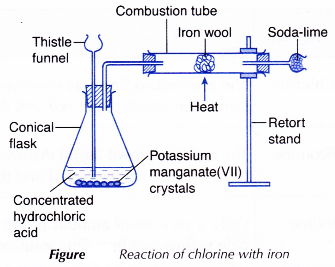
- The iron wool is heated strongly until it is red-hot.
- Concentrated hydrochloric acid is then poured onto the potassium manganate(VII) crystals through the thistle funnel until the other end of the thistle funnel is submerged in the concentrated hydrochloric acid.
- The liberated chlorine gas is passed over the red-hot iron wool in the combustion tube until no further change occurs.
- All the changes are recorded.
II. Bromine with iron
- The arrangement of apparatus as shown in Figure is set up.
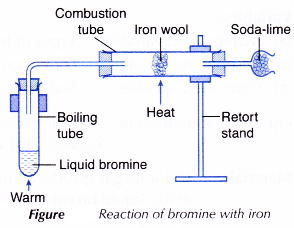
- The iron wool is heated strongly until it is red-hot.
- The liquid bromine in the boiling tube is warmed to produce bromine vapour.
- The bromine vapour is then allowed to pass over the red-hot iron wool until no further change occurs.
- All the changes are recorded.
III. Iodine with iron
- The arrangement of apparatus as shown in Figure is set up.
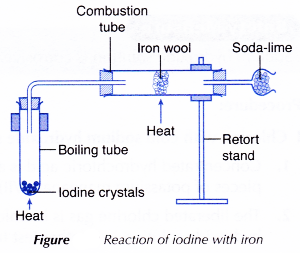
- The iron wool is heated strongly in the combustion tube until it is red-hot.
- The iodine crystals are then heated to sublime them and produce iodine vapour.
- The liberated iodine vapour is passed over the red-hot iron wool until no further change occurs.
- All the changes are recorded.
Observations:
| Halogen | Observation |
| Chlorine | The hot iron wool ignites rapidly with a bright flame. A brown solid is formed. |
| Bromine | The hot iron wool glows moderately bright, moderately fast and less vigorously. A brown solid is formed. |
| Iodine | The hot iron wool glows dimly and slowly. A brown solid is formed. |
Discussion:
- Chlorine, bromine and iodine react with hot iron to produce a brown solid. Hence, chlorine, bromine and iodine exhibit similar chemical properties.
- The observations also show that the reactivity of the halogens in their reactions with iron decreases from chlorine → bromine → iodine.
- Halogens react with hot iron to produce iron(lll) halides (brown salts).

- Soda lime is used to absorb the excess poisonous chlorine gas, bromine vapour or iodine vapour. This will prevent the poisonous gases from escaping to the surroundings.
Note: Soda lime is a solid mixture of calcium hydroxide and sodium hydroxide.
C. Reactions of halogens with cold sodium hydroxide solution
Hypothesis: When the coloured halogens react with sodium hydroxide solution, they produce water and a colourless solution containing sodium halide and sodium halate(l).
Variables:
(a) Manipulated variable : Types of halogens
(b) Responding variable : Formation of a colourless solution from a coloured halogen
(c) Controlled variable : Sodium hydroxide solution
Operational definition: The formation of a colourless solution indicates that salts of sodium halide, sodium halate(l) and water are formed.
Materials: Chlorine gas (produced by mixing potassium manganate(VII) crystals with concentrated hydrochloric acid), liquid bromine, solid iodine and 2 mol dm-3 sodium hydroxide solution.
Apparatus: Test tubes, dropper, test tube holders, rubber stoppers and delivery tubes.
Procedure:
I. Chlorine with cold sodium hydroxide solution
- Concentrated hydrochloric acid is added just enough to cover a few pieces of potassium manganate(VII) crystals in a test tube.
- The liberated chlorine gas is bubbled through 2 cm3 of cold sodium hydroxide solution in another test tube, as shown in Figure.
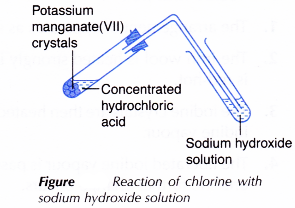
- The test tube is shaken strongly.
- All the changes are recorded.
II. Bromine with cold sodium hydroxide solution
- Two drops of liquid bromine are added to 2 cm3 of cold sodium hydroxide solution in a test tube, as shown in Figure.
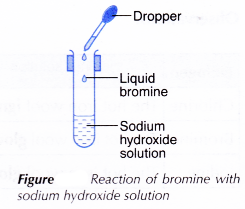
- The test tube is closed tightly with a rubber stopper and shaken vigorously until no further change occurs.
- The changes are recorded.
III. Iodine with cold sodium hydroxide solution
- A small piece of iodine crystal is added to 2 cm3 of cold sodium hydroxide solution in a test tube.
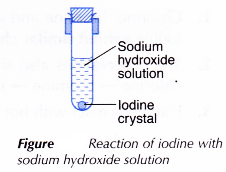
- The test tube is closed tightly with a rubber stopper and shaken vigorously until no further change occurs.
- The changes are recorded.
Observations:
| Halogen | Observation |
| Chlorine | The greenish-yellow gas dissolves rapidly in sodium hydroxide solution to produce a colourless solution. |
| Bromine | The reddish-brown liquid dissolves moderately fast in sodium hydroxide solution to produce a colourless solution. |
| Iodine | The purplish-black solid dissolves slowly in sodium hydroxide solution to produce a colourless solution. |
Discussion:
- The reactivity of halogens in their reactions with cold sodium hydroxide solution decreases from chlorine → bromine → iodine (down Croup 1 7).
- The halogens react with cold sodium hydroxide solution to produce water and a colourless solution containing salts of sodium halide and sodium halate(l).

Note: Sodium chlorate(l), sodium bromate(l) and sodium iodate(l) are also known as sodium hypochlorite, sodium hypobromite and sodium hypoiodite respectively.
Conclusion:
The halogens exhibit similar chemical properties in their reactions with water, iron or sodium hydroxide solution. The reactivity of halogens decreases down Group 17. The hypothesis proposed can be accepted.
