Physical and Chemical Properties of Group 1 Elements
Group 1 Elements: The Alkali Metals
The elements in Group 1 are:

These elements are known as alkali metals.
Physical Properties of Group 1 Elements
1. Table shows some properties of Group 1 elements.
| Element | Proton number | Nucleon number | Density (g cm-3) | Hardness (Brinell) | Melting point (°C) | Boiling point (°C) | Atomic radius (nm) | Electro negativity |
| Lithium | 3 | 7 | 0.53 | 0.06 | 181 | 1347 | 0.15 | 1.0 |
| Sodium | 11 | 23 | 0.97 | 0.07 | 98 | 886 | 0.19 | 0.9 |
| Potassium | 19 | 39 | 0.86 | 0.04 | 64 | 774 | 0.23 | 0.8 |
| Rubidium | 37 | 85 | 1.53 | 0.03 | 39 | 688 | 0.25 | 0.8 |
| Caesium | 55 | 133 | 1.87 | 0.02 | 28 | 678 | 0.26 | 0.7 |
| Francium | 87 | 223 | 2.40 | ? | 27 | 677 | 0,29 | 0.7 |
2. General physical properties of Group 1 elements:
- Alkali metals are grey solids with shiny silvery surfaces when freshly cut.
- These surfaces turn dull when exposed to air.
- This is because alkali metals are very reactive. They react rapidly with oxygen and water vapour in the air when exposed.
- Alkali metals are soft solids and can be easily cut.
- Alkali metals have low densities as compared to heavy metals such as iron and copper.
- Alkali metals are good conductors of heat and electricity.
- Alkali metals have low melting and boiling points as compared to heavy metals such as copper and iron.
Table compares the melting and boiling points of potassium (an alkali metal) and copper (a heavy metal).
| Element | Potassium | Copper |
| Melting point (°C) | 64 | 1083 |
| Boiling point (°C) | 774 | 2567 |
3. Trend of change in the physical properties
The physical properties of the elements vary gradually when going down Group 1 as shown in Table.
| Group 1 elements | Trend of change in the physical properties | |||
 | The atomic radius (atomic size) of alkali metals increases gradually down the group. Reason: | Although alkali metals have low densities, the densities increase gradually down the group. For example: Metallic bond means the chemical bond that holds the atoms together in a metal. | Although alkali metals have low melting and boiling points, the melting and boiling points decrease gradually down the group. Reason: | Alkali metals become softer down the group. |
People also ask
- What is the periodic table of the elements?
- Physical and Chemical Properties of Group 18 Elements
- Physical and Chemical Properties of Group 17 Elements
- What do you mean by transition metals?
- What is the Need for Classification of Elements?
- Modern Periodic Table and Its Significance
- How did Mendeleev Arrange the Periodic Table?
- Periodic Trends in Properties of Elements
- What is Valency and Atomic Radius?
- What are Metallic and Nonmetallic Properties?
Chemical Properties of Group 1 Elements
1. Table shows the electron arrangements of alkali metals.
| Element | Electron arrangement |
| Lithium | 2.1 |
| Sodium | 2.8.1 |
| Potassium | 2.8.8.1 |
| Rubidium | 2.8.18.8.1 |
| Caesium | 2.8.18.18.8.1 |
| Francium | 2.8.18.32.18.8.1 |
2. Similar chemical properties
- All alkali metal exhibit similar chemical properties.
- This is because all the atoms of alkali metals have one valence electron.
3. Reactivity
Alkali metals are very reactive.
Although alkali metals exhibit similar chemical properties, they differ in reactivity.
The reactivity of alkali metals increases when going down Group 1.
![]()
The reactivity of an alkali metal is measured by how easily its atom loses its single valence electron to achieve a stable noble gas electron arrangement (duplet or octet electron arrangement).
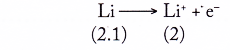
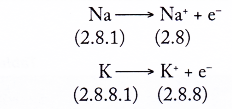
The easier an alkali metal atom releases its single valence electron, the more reactive is the alkali metal.
Explanation:
The increase in reactivity of alkali metals down Group 1 can be explained as follows.
- All alkali metals have one valence electron.
- Each atom of an alkali metal will release one valence electron during a chemical reaction to achieve a stable duplet or octet electron arrangement. Hence, an ion with a charge of+1 is formed.
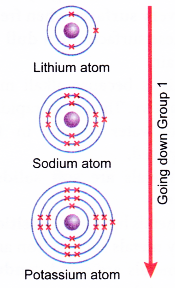
- When going down Group 1, the atomic size of alkali metals increases.
- The single valence electron becomes further away from the nucleus and is screened by more inner shells containing electrons.
- This means that the effective nuclear charge felt by the single valence electron decreases when going down the group.
- This causes the attractive forces between the nucleus and the single valence electron become weaker, so the single valence electron is more weakly pulled by the nucleus.
- Hence, the single valence electron can be released more easily when going down the group.
- As a result, the reactivity of alkali metals increases down the group.
4. As reducing agents
- Reducing agents are good electron donors in chemical reactions.
- Alkali metals are good reducing agents because the single valence electron in the atom of each alkali metal can be easily released to achieve a stable electron arrangement of a noble gas (good electron donor).
- The strength of alkali metals as reducing agents increases when going down Group 1.

- This is because the single valence electron of the alkali metals becomes much easier to be released when going down the group.
- The strength of alkali metals as reducing agents increases when going down Group 1.
5. Electropositivity
- (a) Definition:
Electropositivity of an element is a measurement of the ability of an atom to donate electrons to form a positive ion.- Alkali metals are very electropositive.
- This is because the atom of each alkali metal can release its single valence electron easily to form a positive ion.
- However, the electropositivity of alkali metals increases when going down Group 1.

This can be explained as below:
- The atomic size of alkali metals increases from lithium to francium.
- The single valence electron in the outermost occupied shell becomes further away from the nucleus and is screened by more inner shells containing electrons.
- So, the attractive forces between the nucleus and the single valence electron become weaker when going down Group 1.
- This causes the single valence electron to be released more easily when going down Group 1.
- As a result, the electropositivity of alkali metals increases when going down the group.
6. Group 1 elements exhibit similar chemical properties in their reactions with
- water to liberate hydrogen gas and form metal hydroxide.
- oxygen to produce metal oxides.
- chlorine to produce metal chloride.
- bromine to produce metal bromide.
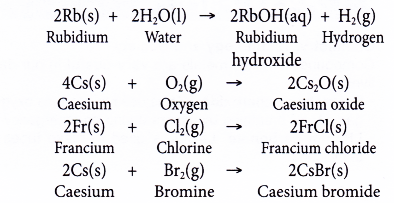
7. To predict the properties of rubidium, caesium and francium
- Rubidium, caesium and francium are placed below potassium in Group 1 of the Periodic Table.
- Hence, rubidium, caesium and francium are expected to react with water, oxygen, chlorine or bromine in a similar way as potassium but these reactions are more vigorous (more reactive) than potassium.
- For example:
8. Solubility of the salts of alkali metals
- Carbonate, nitrate, chloride, sulphate, bromide and iodide salts of alkali metals are white solids.
- These salts are soluble in water. They dissolve in water to form colourless solutions.
9. Safety precautions in handling Group 1 elements
- Alkali metals are very reactive.
- Alkali metals, when exposed, can react with oxygen and water vapour in the air.
- Hence, alkali metals such as lithium, sodium and potassium must be kept in paraffin oil, whereas rubidium and caesium are stored in sealed glass tubes. This is to prevent them from reacting with oxygen and water vapour in the air.
- The following safety precautions must be taken when handling alkali metals.
- Avoid holding the highly reactive alkali metals with your bare hands.
- Wear safety goggles and gloves during an experiment.
Chemical Properties of Group 1 Elements Experiment 1
Aim: To investigate the chemical properties of Group 1 metals in their reactions with water and oxygen.
Problem statement: How do Group 1 metals react with water and oxygen?
A. Reactions of alkali metals with water
Hypothesis: When going down Group 1, alkali metals become more reactive in their reactions with water.
Variables:
(a) Manipulated variable : Different types of alkali metals
(b) Responding variable : Reactivity of alkali metals
(c) Controlled variables : Water, size of alkali metals
Operational definition: An alkali metal that reacts more vigorously and rapidly with water is a more reactive metal. Materials: Small pieces of lithium, sodium and potassium, distilled water, red litmus paper and filter paper.
Apparatus: Water troughs, small knife and forceps.
Safety Measure:
Do not touch the extremely reactive alkali metals with your bare hands.
Always wear safety goggles and gloves.
Procedure:
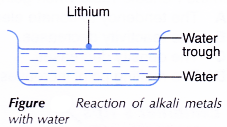
A. small piece of lithium is cut out using a knife.
- The oil on the surface of lithium is removed by rolling it on a piece of filter paper.
- The lithium is then placed slowly onto the water surface in a water trough with the help of forceps, as shown in Figure.
- All changes that occur are recorded.
- When the reaction stops, the solution formed is tested with a piece of red litmus paper.
- Steps 1 to 5 are repeated using sodium and potassium respectively to replace lithium.
Observations:
| Alkali metal | Observation |
| Lithium | Lithium moves slowly on the water surface with a soft ‘hiss’ sound. A colourless solution that turns red litmus paper blue is formed. |
| Sodium | Sodium melts to become a small sphere, moves rapidly and randomly on the water surface with a hiss’ sound. A colourless solution that turns red litmus paper blue is formed. |
| Potassium | Potassium melts to become a small sphere, burns with a lilac flame, moves very rapidly and randomly on the water surface with ‘hiss’ and ‘pop’ sounds. A colourless solution that turns red litmus paper blue is formed. |
Discussion:
- The alkali metals fizz and push around on the water surface like a hovercraft. This is due to the liberation of hydrogen gas as they react with water.
- Lithium, sodium and potassium react with water to produce a colourless gas (‘hissing’ sound) and an alkaline solution (metal hydroxide) that turns red litmus paper blue. Hence, lithium, sodium and potassium exhibit similar chemical properties.
- The observations also show that the reactivity of the alkali metals in their reactions with water increases from lithium → sodium → potassium.
- Alkali metals react with water to produce a metal hydroxide solution (an alkaline solution) and hydrogen gas.

B. Reactions of alkali metals with oxygen
Hypothesis: When going down Group 1, alkali metals become more reactive in their reactions with oxygen.
Variables:
(a) Manipulated variable : Different types of alkali metals
(b) Responding variable : Reactivity of alkali metals
(c) Controlled variables : Oxygen gas, size of alkali metals
Operational definition: An alkali metal that burns more rapidly and vigorously in oxygen gas is a more reactive metal.
Materials: Small pieces of lithium, sodium and potassium, filter paper, red litmus paper and three gas jars filled
with oxygen gas.
Apparatus: Forceps, gas jar spoon, small knife and Bunsen burner.
Procedure:
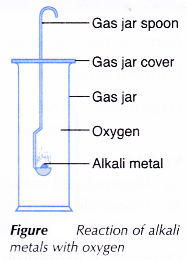
- A small piece of lithium is cut out using a knife.
- The oil on the surface of lithium is removed by roiling it on a piece of filter paper.
- The lithium is then heated in a gas jar spoon until it starts to burn.
- The gas jar spoon with the burning lithium is then quickly lowered into a gas jar filled with oxygen gas, as shown in Figure.
- The changes that occur are recorded.
- When the reaction stops, 10 cm3 of distilled water is poured into the gas jar and shaken well.
- The solution formed is then tested with a piece of red litmus paper.
- Steps 1 to 7 are repeated using sodium and potassium respectively to replace iithium.
Observations:
| Alkali metal | Observation |
| Lithium | Lithium burns slowly with a red flame and liberates white fumes which become a white solid on cooling to room temperature. The white solid dissolves in water to produce a colourless solution. This solution turns red litmus paper blue. |
| Sodium | Sodium burns rapidly and brightly with a yellow flame and liberates white fumes which become a white solid on cooling to room temperature. The white solid dissolves in water to produce a colourless solution. This solution turns red litmus paper blue. |
| Potassium | Potassium burns very rapidly and brightly with a lilac flame and liberates white fumes which become a white solid on cooling to room temperature. The white solid dissolves in water to produce a colourless solution. This solution turns red litmus paper blue. |
Discussion:
- Lithium, sodium and potassium burn in oxygen gas respectively to produce white fumes which then become a white solid (metal oxide). The white solid dissolves in water to form an alkaline solution (metal hydroxide). Hence, it can be inferred that these alkali metals exhibit similar chemical properties.
- From the brightness of the flame and the speed of burning, it can be inferred that the reactivity of the alkali metals in their reactions with oxygen gas increases from lithium → sodium → potassium.
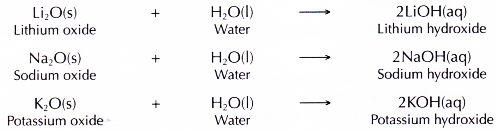
- All the alkali metals react with oxygen gas when heated to produce white solid metal oxides.

- The white solid metal oxides formed dissolve in water to produce metal hydroxide solutions which are alkaline.
Conclusion:
The alkali metals exhibit similar chemical properties in their reactions with water or oxygen gas. The reactivity of alkali metals increases down Group 1. Hence, the hypothesis proposed can be accepted.
Chemical Properties of Group 1 Elements Experiment 2
Aim: To investigate the chemical properties of Group 1 metals in their reactions with chlorine and bromine.
Problem statement: How do Group 1 metals react with chlorine and bromine?
Hypothesis: When going down Group 1, alkali metals become more reactive in their reactions with chlorine or bromine.
Variables:
(a) Manipulated variable : Different types of alkali metals
(b) Responding variable : Reactivity of alkali metals
(c) Controlled variables : Chlorine and bromine, size of alkali metals
Operational definition: An alkali metal that reacts more vigorously and rapidly with chlorine or bromine gas is a more reactive metal.
Materials: Small pieces of lithium, sodium and potassium, filter paper, three gas jars filled with chlorine gas and three gas jars filled with bromine vapour.
Apparatus: Bunsen burner, forceps, gas jar spoon and small knife.
Safety Measures:
Chlorine gas and bromine vapour are poisonous. Wear gloves and safety goggles when handling these halogens.
Procedure:
- A small piece of lithium is cut out using a knife.
- The oil on the surface of lithium is removed by rolling it on a piece of filter paper.
- The lithium is then heated in a gas jar spoon until it starts to burn.
- The gas jar spoon with the burning lithium is then quickly lowered into a gas jar filled with chlorine gas, as shown in Figure.
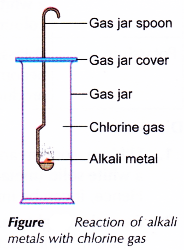
- The changes that occur are recorded.
- Steps 1 to 5 are repeated using sodium and potassium respectively to replace lithium.
- Steps 1 to 6 are repeated using bromine vapour to replace chlorine gas.
Observations:
| Alkali metal | Observation | |
| Chlorine gas | Bromine vapour | |
| Lithium | Lithium burns slowly with a red flame and liberates white fumes which become a white solid at the end of the reaction. | Lithium burns slowly with a red flame and liberates white fumes which become a white solid at the end of the reaction. The reddish-brown bromine vapour is decolourised. |
| Sodium | Sodium burns rapidly and brightly with a yellow flame and liberates white fumes which become a white solid at the end of the reaction. | Sodium burns rapidly and brightly with a yellow flame and liberates white fumes which become a white solid at the end of the reaction. The reddish-brown bromine vapour is decolourised. |
| Potassium | Potassium burns very rapidly and brightly with a lilac flame and liberates white fumes which become a white solid at the end of the reaction. | Potassium burns very rapidly and brightly with a lilac flame and liberates white fumes which become a white solid at the end of the reaction. The reddish-brown bromine vapour is decolourised. |
Discussion:
- Lithium, sodium and potassium exhibit similar chemical properties in their reactions with chlorine gas or bromine vapour. This is because all these alkali metals have one valence electron.
- From the brightness of the flame and the speed of burning, it can be inferred that the reactivity of alkali metals in their reactions with chlorine or bromine increases from lithium → sodium → potassium.
- All alkali metals react with chlorine gas when heated to produce white solid metal chlorides.
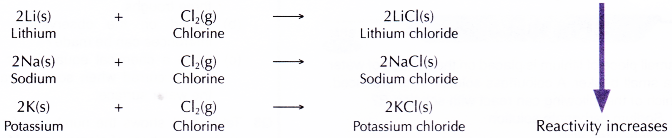
- Alkaliali metals react with brorr ine vapour when heated to produce white solid metal bromides.
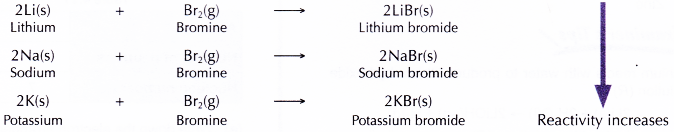
Conclusion:
The alkali metals exhibit similar chemical properties in their reactions with chlorine gas or bromine vapour. The reactivity of alkali metals increases down Group 1. Hence, the hypothesis proposed can be accepted.
