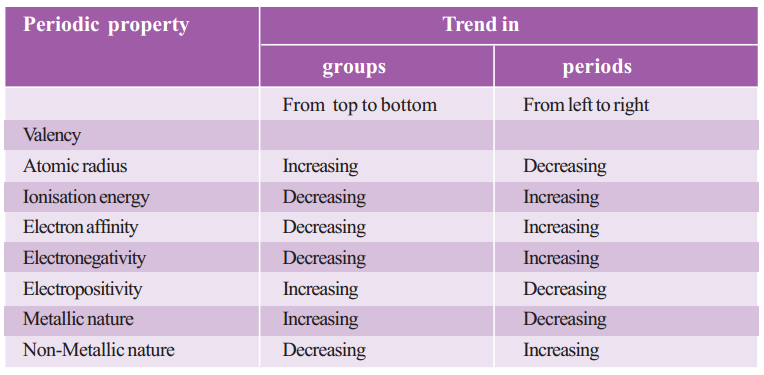
Periodic Trends in Properties of Elements
Periodicity in Properties :
The properties of elements depends upon the electronic configuration which changes along a period and down a group in periodic table.
There is periodicity in properties, i.e., repetition of properties after a regular interval due to similarity in electronic configuration.
Atomic Size (Atomic radii) :
Atomic size means radius of an atom. It is defined as distance between centre of nucleus and outermost shell (valence shell) of an isolated atoms.
Covalent Radii :
It is defined as half of the distance between the centres of nuclei of two atoms (bond length) bonded by a single covalent bond, e.g., Bond length in case of H—H (Hydrogen molecule) is 74 pm.
Covalent radius = 1/2 × 74 pm = 37 pm (picometre) [1 pm = 10–12 m]
It can be measured in case of diatomic molecules of non-metals.
Metallic Radii : If is defined as half of the internuclear distance between the two metal ions in a metallic crystal. It is measured in case of metals.
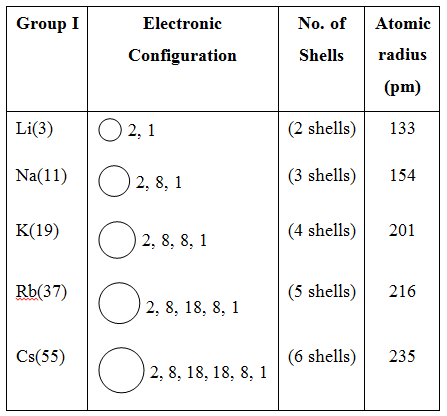
Variation of Atomic size in a Group :
Size generally increases from top to bottom in a group.
Reason : It is due to addition of a new shell, i.e., number of shells go one increasing, e.g., pm stands for picometre, i.e., 10–12 m.
People also ask
- What is the periodic table of the elements?
- Physical and Chemical Properties of Group 1 Elements
- Physical and Chemical Properties of Group 18 Elements
- Physical and Chemical Properties of Group 17 Elements
- What do you mean by transition metals?
- What is the Need for Classification of Elements?
- Modern Periodic Table and Its Significance
- How did Mendeleev Arrange the Periodic Table?
- What is Valency and Atomic Radius?
- What are Metallic and Nonmetallic Properties?
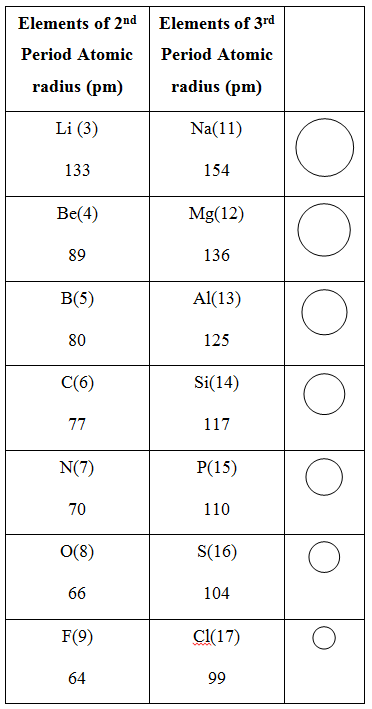
Variation of Atomic size along a Period :
Atomic size goes on decreasing along a period from left to right
Reason : It is due to increase in nuclear charge (number of protons in nucleus) which pulls the electrons towards it, i.e., force of attraction between nucleus and valence electrons increase, therefore atomic size decreases, e.g.,
Ionisation Energy and Electron Affinity :
Chemical nature and reactivity of an element depend upon the ability of its atoms to donate or accept electrons. This can be measured quantitatively with the help of ionisation energy and electron affinity of its atoms :
Ionisation energy : It is defined as the energy required to remove an electron completely from an isolated gaseous atom of an element. The energy required to remove the 1st electron is called first ionisation energy.
A(g) + I.E1 → A+(g) + e–
Second Ionisation Energy : he energy required to remove an electron from a unipositive ion is called the second ionisation energy.
A+(g) + I.E2 → A2+ (g) + e–
Te second ionisation energy is greater than the first ionisation energy due to increase in positive charge, hence increase in force of attraction between the nucleus and the valence electron.
Ist I.E. < 2nd I.E. < 3rd I.E.
Variation of Ionisation energy down a Group :
Ionisation energy goes on decreasing down a group.
Reason : It is due to the increase in the distance between the valence electrons and the nucleus as the atomic size increase down a group, the force of attraction between the nucleus and the valence electrons decrease, therefore, the energy required to remove the electrons, i.e., the ioisation energy goes on decreasing
Example :
Group I | Ionisation Energy (in kJ mol–1) | Group 2 | Second Ionisation Energy (in kJ mol–1) | First Ionisation Energy (in kJ mol–1) |
Li Na K Rb Cs | 500 496 420 403 376 | Be Mg Ca Sr Ba | 1757 1450 1146 1064 965 | 899 737 590 549 503 |
Variation of Ionisation energy along a Period :
It goes on increasing generally along a period from left to right with decrease in atomic size.
Reason : Due to decrease in atomic size, the force of attraction between the valence electrons and the nucleus increase and, therefore, more energy is required to remove electron.
Example :
Elements of 2nd Period | I.E. in kJ mol–1 |
| Li Be B C N O F Ne | 500 900 801 1085 1400 1314 1680 2080 |
There is a decrease in ionisation energy from Be to B and from N to O, the reason of which you will study in higher classes.
Group 18 elements (noble gases) have the highest ionisation energy in respective periods due to stable electronic configuration, i.e., 8 electrons in their valence shells except He which has 2 electrons.
Electron Affinity :
It is the energy change when an electron is gained by a neutral gaseous atom converting it into a negatively charged ion.
It is a measure of attraction or affinity of the gaseous atom for an extra electron to be added.
Cl(g) + e– → Cl–(g) + E.A.
Factors :
It depends upon atomic size as well as electronic configuration.
Variation down the Group :
Electron affinity goes on decreasing down the group in general.
Reason : Due to the increase in atomic size, the force of attraction between the nucleus and the electron to e added becomes less.
Variation along a Period :
Electron affinity increase from left to right in period.
Reason : It is due to decrease in atomic size which leads to an increase in the force of attraction between the nucleus and the electrons to be added.
Example :
Group 17 | E.A. (kJ mol–1) |
| F Cl Br I | 333 348 324 295 |
However, deviations to this rule are observed in variation of electron affinity.