Oxidation and Reduction in Chemical Cells
- In a simple voltaic cell, two different metals are in contact with an electrolyte.
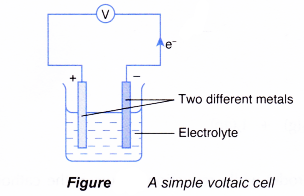
- The more electropositive metal will become the negative terminal while the less electropositive metal will become the positive terminal.
- In a simple voltaic cell, two different metals are in contact with an electrolyte.
- The chemical change that takes place at each electrode is actually a half-reaction of a redox reaction. As a result of the redox reaction, a flow of electrons or an electric current is produced.
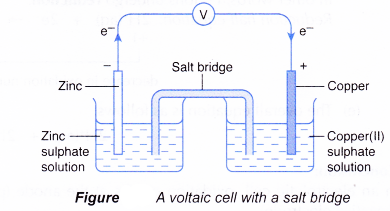
- The set-up of a simple voltaic cell may also include a salt bridge or a porous pot. The salt bridge or porous pot separates the half-reactions while completing the circuit by allowing the movement of ions to take place.
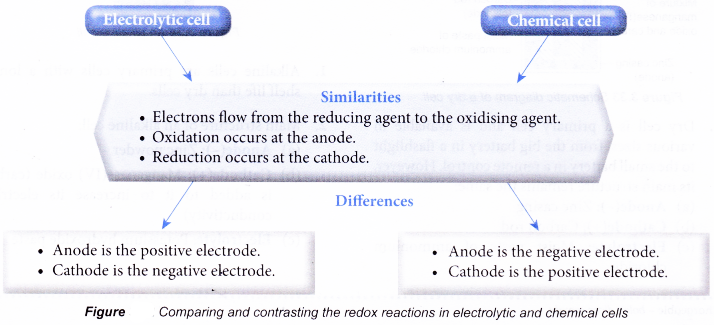
- Figure compares and contrasts the redox reactions in electrolytic and chemical cells.
People also ask
- Electrolytic and Chemical Cells
- Oxidation and Reduction in Electrolytic Cells
- What is a redox reaction?
- Changing of iron(II) ions to iron(III) ions and vice versa
- Redox reaction in the displacement of metals from its salt solution
- Displacement of Halogen From Halide Solution
- Redox Reactions by Transfer of Electrons at a Distance
- Rusting as a Redox Reaction
- The Reactivity Series of Metals Towards Oxygen
- Application of the reactivity series of metals in the extraction of metals
- How does a voltaic cell work?
Oxidation and Reduction in Chemical Cells Experiment
Aim: To investigate the oxidation and reduction in chemical cells.
Materials: 1 mol dm-3 copper(II) sulphate solution, 1 mol dm-3 zinc sulphate solution, copper strip, zinc strip, sandpaper.
Apparatus: Porous pot, voltmeter, connecting wires with crocodile clips, beaker.
Procedure:
- Zinc sulphate solution is poured into a porous pot until three quarters full.
- The porous pot is placed in a beaker.
- Copper(II) sulphate solution is poured into the beaker until its level is the same as that of the solution in the porous pot.
- A strip of copper and a strip of zinc are cleaned with sandpaper.
- The two strips are connected as shown in Figure.
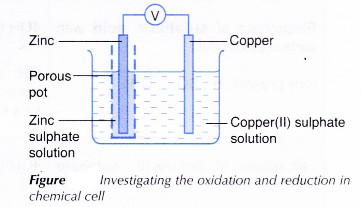
- The set-up of the apparatus is left aside for 20 minutes. Any changes are observed.
Observations:
- The voltmeter shows a reading.
- The deflection of the indicator of the voltmeter indicates that electric current flows from the copper strip to the zinc strip.
- The intensity of the blue colour of the copper(II) sulphate solution decreases with time.
- Brown solid is deposited on the copper strip.
- The zinc strip becomes thinner.
Discussion:
- Since electric current flows from the copper strip to the zinc strip, it is inferred that electrons flow from the zinc strip to the copper strip.
Note: Conventionally, electrons flow in the opposite direction of electric current. - This means that the zinc strip becomes the negative terminal while the copper strip becomes the positive terminal.
- (a) Zinc is more electropositive than copper. In other words, zinc can lose its electrons more readily than copper.
(b) Therefore, zinc acts as the reducing agent, losing electrons to form zinc ions, Zn2+. This explains why the zinc strip becomes thinner.
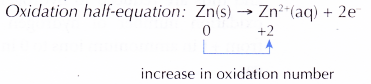
(c) The accumulated electrons cause the zinc strip to become the negative terminal.
(d) By losing its electrons, zinc undergoes oxidation. Thus, the zinc strip also serves as the anode. - The accumulated electrons then flow out of the zinc strip through the connecting wires to the copper strip. This makes the copper strip the positive terminal.
- At the positive terminal, copper(II) ions, Cu2+ from the electrolyte act as the oxidising agent by accepting the electrons. By doing so, Cu2+ ions are reduced to metallic copper.
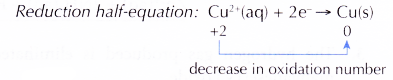
- As reduction occurs at the copper strip, the copper strip is said to serve as the cathode.
- (a) Due to the decrease in the amount of Cu2+ ions in the solution, the intensity of the blue colour of the copper(II) sulphate solution slowly decreases.
(b) Metallic copper that is produced forms a brown layer around the strip. - In this chemical cell, electrons flow from zinc, the reducing agent at the anode or negative terminal, to Cu2+ ions, the oxidising agent at the cathode or positive terminal.
Conclusion:
In a chemical cell, oxidation occurs at the anode (negative terminal) while reduction occurs at the cathode (positive terminal).
Follow us on:
https://plus.google.com/+Veerendracbse
https://plus.google.com/+DurgaprasannaAmujuri
https://plus.google.com/+phanicbse
https://plus.google.com/+vinilachoudary
https://plus.google.com/+LearnCbseind
https://plus.google.com/+kajalsharmaLearnCBSE
https://plus.google.com/+sravyasharma
https://plus.google.com/+kajalsharmaLearnCBSE
https://plus.google.com/+cbseguesspapersfree
https://plus.google.com/+CharanSing
https://plus.google.com/+rajuheal
https://plus.google.com/+saralasharma
https://plus.google.com/+mastiaddaLearnCBSE
https://plus.google.com/+ChandraRamLearnCBSE
https://plus.google.com/+AmrutPandey
https://plus.google.com/+VenkataLakshmiLearnCBSE
https://plus.google.com/+nagaRajuLearnCBSE
https://plus.google.com/+SekharThe
https://plus.google.com/+TriveniMcc
