How do you know the Order of Elements in a Chemical Formula
What is Chemical Formula?
A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present in the substance.
Based on the chemical formula of a substance, we know the composition of the substance.
(a) The elements that make up the substance.
(b) The ratio or number of atoms of each element in the substance.
Chemical formulae of elements
The chemical formula of an element is a statement of the composition of its molecule in which symbol tells us the element and the subscript tells us how many atoms are present in one molecule. One molecule of hydrogen element contains two atoms of hydrogen, therefore, the formula of hydrogen is H2.
- For elements that exist as atoms, their chemical formulae represent their atoms.
- For elements that exist as molecules, their chemical formulae represent their molecules. Subscript number at the right bottom shows the number of atoms in each molecule.
Example: The formula H2 indicates that one molecule of hydrogen element contains 2 atoms of hydrogen. 2 H represents 2 separate atoms of hydrogen; H2 represents 1 molecule of hydrogen and 2H2 represents 2 molecules of hydrogen.
Table: Chemical formulae of some elements
| Element | Chemical Formula | Meaning of chemical formula |
| Carbon | C | Carbon is made up of carbon atoms. |
| Argon | Ar | Argon is made up of. argon atoms. |
| Copper | Cu | Copper is made up of copper atoms. |
| Oxygen gas | O2 | Each oxygen molecule consists of two oxygen atoms. |
| Chlorine gas | Cl2 | Each chlorine molecule consists of two chlorine atoms. |
People also ask
- What is the Relative Atomic Mass and Relative Molecular Mass of an Element?
- What is One Mole and How many Particles are in a Mole?
- How do you Calculate the Molar Mass of a Substance?
- What is the Molar Volume of a Gas at STP?
- What is Empirical and Molecular Formula?
- How do you Write a Chemical Equation?
Chemical formulae of compounds
- Chemical formula of a compound tells us the composition of the compound.
- However, the chemical formula does not show the type of particles in the compound.
- Example: Water is a compound made up of 2 atoms of hydrogen element and 1 atom of oxygen element, so the formula of water is written as H2O. In the formula H2O, the subscript 2 indicates 2 atoms of hydrogen. In the formula of water, oxygen O is written without a subscript and it indicates 1 atoms of oxygen.
Table: Chemical formulae of some compounds
| Compound | Chemical formula | Meaning of chemical formula | ||
| Elements present | Ratio of the number of atoms of elements | |||
| Water | H2O | Hydrogen, oxygen | H:O = 2:1 | |
| Ammonia | NH3 | Nitrogen, hydrogen | N:H = 1:3 | |
| Sulphuric acid | H2SO4 | Hydrogen, sulphur, oxygen | H:S:O = 2:1:4 | |
| Zinc hydroxide | Zn(OH)2 | Zinc, oxygen, hydrogen | Zn:O:H = 1:2:2 | |
| Magnesium nitrate | Mg(NO3)2 | Magnesium, nitrogen, oxygen | Mg;N;O = 1:2:6 | |
Rules for writing a chemical formula
- We first write the symbols of the elements which form the compound
- Below the symbol of each element, we write down its valency.
- Finally, we cross-over the valencies of the combining atoms. That is, with first atom we write the valency of second atom (as a subscript); and with the second atom we write the valency of first atom (as subscript).
Example 1:
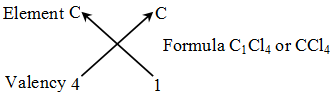
Example 2:
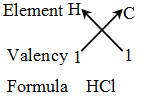
Example 3:
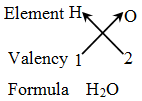
Example 4:
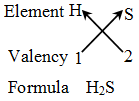
Example 5:
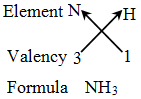
Writing the formula of a compound
Step-1 :
Write the symbols of formulae of the ions of the compound side by side with positive ion on the left hand side and negative ion on right hand side.
Step-2 :
Enclose the polyatomic ion in a bracket.
Step-3 :
Write the valency of each ion below its symbol
Step-4 :
Reduce the valency numerals to a simple ratio by dividing with a common factor, if any.
Step-5 :
Cross the valencies. Do not write the charges positive or negative of the ions.
Example: Formula of barium nitrate.
Step-1 : Writing the formula of the ions :
Ba2+ NO3–
Step-2 : Ba2+ (NO3)–
Step-3 : Ba2+ (NO3)–
2 1
Step-4 : Not applicable, because ratio is already simple
Step-5 :
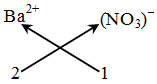
Thus, the formula of barium nitrate is Ba(NO3)2
Significance of the formula of a substance
- Formula represents the name of the substance.
- Formula represents one molecule of the substance.
- Formula also represents one mole of molecules of the substance. That is, formula also represents 6.022 × 1023 molecules of the substance.
- Formula gives the name of all the elements present in the molecule.
- Formula gives the number of atoms of each element present in one molecule.
- Formula represents a definite mass of the substance.
Chemical formulae of ionic compounds
- Ionic compounds consist of cations and anions.
(a) Cations are positively-charged ions.
(b) Anions are negatively-charged ions. - To construct chemical formulae of ionic compounds, we need to know the formulae of cations and anions.
- Below tables show the formulae of some common cations and anions.
Table: Formulae of some common cations.Charge of cation Cation (positive ion) Formula of cation + 1 Sodium ion Na+ Potassium ion K+ Silver ion Ag+ Hydrogen ion H+ Ammonium ion NH4+ Copper(I) ion Cu2+ +2 Calcium ion Ca2+ Magnesium ion Mg2+ Zinc ion Zn2+ Barium ion Ba2+ Iron(II) ion Fe2+ Copper(II) ion Cu2+ Tin(II) ion Sn2+ Lead(II) ion Pb2+ +3 Iron(III) ion Fe3+ Aluminium ion Al3+ Chromium(III) ion Cr3+ +4 Tin(IV) ion Sn4+ Lead(IV) ion Pb4+ Charge of anion Anion
(negative ion)Formula of anion -1 Fluoride ion F– Chloride ion cl– Bromide ion Br– Iodide ion I– Hydroxide ion OH– Nitrate ion NO3– Nitrite ion NO2– Hydride ion H– Ethanoate ion CH3COO– Manganate(VII) ion MnO4– Chlorate(I) ion ClO– Bromate(I) ion BrO– Iodate(I) ion IO– Chlorate(V) ion ClO3– Bromate(V) ion BrO3– Iodate(V) ion IO3– -2 Oxide ion O2– Carbonate ion CO32- Sulphide ion S2- Sulphate ion SO42– Sulphite ion S2O32- Thiosulphate ion O32– Chromate(VI) ion CrO42- Dichromate(VI) ion Cr2O72- -3 Phosphate ion PO43- - Even though ionic compounds contain charged particles, their chemical formulae are electrically neutral. This is because the total positive charges are equal to the total negative charges.
- Steps in constructing the chemical formula of an ionic compound:
- From its name, write the formulae of its cation and anion.
- Determine the number of cations and anions by balancing the positive and negative charges.
- Write the number of cations and anions as subscript numbers.
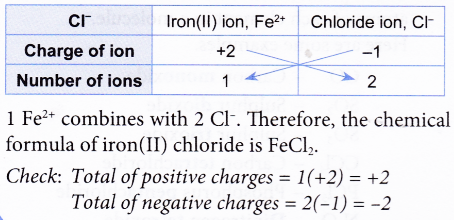
Ex: Construct the chemical formula of iron(II) chloride.
Solution:
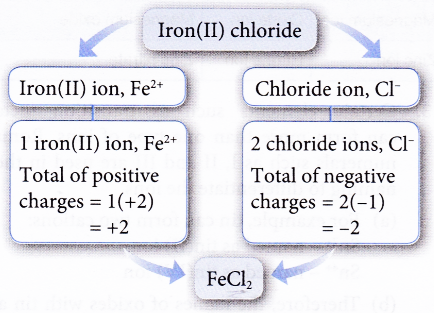
- There is an easier way to determine the number of cations and anions. You only need to interchange the value of the charges of the ions as shown in the following examples.
- However, when the cation and anion have the same value of charges, you need to determine the simplest ratio of the ions.
- Some ions such as OH–, CO32-, SO42– and NO3– are made up of more than one element. Brackets are used to show the number of these ions in formulae.
Table: Usage of brackets in formulaeFormula of ionic compound Usage of brackets in the formula Mg(OH)2 To show that there are 2 groups of OH– AI2(CO3)3 To show that there are 3 groups of CO32- (NH4)2SO4 To show that there are 2 groups of NH4+ Cu(NO3)2 To show that there are 2 groups of NO3–
Chemical formulae of ionic compounds Problems with Solutions
1. Construct the chemical formula of iron(II) chloride.
Solution:
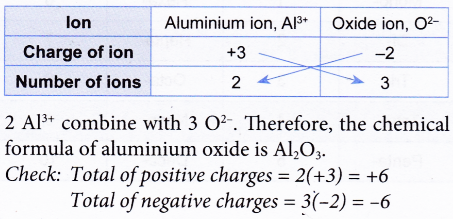
2. Construct the chemical formula of aluminium oxide.
Solution:
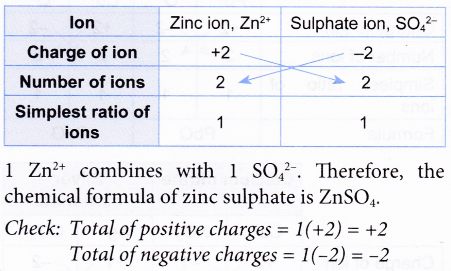
3. Construct the chemical formula of zinc sulphate.
Solution: