How do you Name an Ionic Compound?
Naming of chemical compounds
- Chemical compounds are named systematically according to the guidelines given by the International Union of Pure and Applied Chemistry (IUPAC).
- For ionic compounds, the name of the cation comes first, followed by the name of the anion.
Table: Naming of ionic compoundsCation Anion Name of ionic compound Sodium ion Chloride ion Sodium chloride Magnesium ion Oxide ion Magnesium oxide Zinc ion Nitrate ion Zinc nitrate - Certain elements such as transition metals can form more than one type of ions. Roman numerals such as I, II and III are used in their naming to differentiate the ions.
(a) For example, tin can form two cations:
Sn2+ – named as tin(II) ion
Sn4+ – named as tin(IV) ion
(b) Therefore, the names of oxides with tin are tin(II) oxide and tin(IV) oxide respectively. - For simple molecular compounds, the more electronegative element is written last and is added with an -ide. The name of the first element is maintained as it is. For example, a molecular compound consisting of hydrogen and chlorine is given the name hydrogen chloride. Chlorine is more electronegative than hydrogen.
- Greek prefixes are used to show the number of atoms of each element in a molecule.
Here are some examples.
CO – Carbon monoxide
SO2 – Sulphur dioxide
SO3 – Sulphur trioxide
CCl4 – Carbon tetrachloride
PCl5 – Phosphorus pentachloride
N2O4 – Dinitrogen tetroxide
Cl2O7 – Dichlorine heptoxide - Table below shows the meaning of the prefixes.
Table: Meaning of prefixesPrefix Meaning Prefix Meaning Mono- 1 Hexa- 6 Di- 2 Hepta- 7 Tri- 3 Octa- 8 Tetra- 4 Nona- 9 Penta- 5 Deca- 10
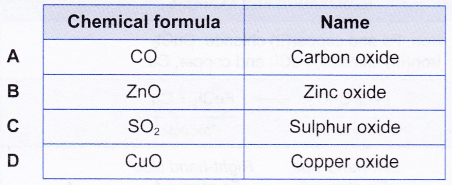
Example: Which chemical formula is correctly named according to the IUPAC nomenclature system?
Solution:
For molecular compounds, prefixes such as ‘mono’ and ‘di’ are used to show the number of atoms of elements in a molecule. Therefore:
For option A, CO should name as carbon monoxide (prefix ‘mono’ represents one atom of oxygen in the molecule).
For option C, SO2 should name as sulphur dioxide (prefix di’ represents two atoms of oxygen in the molecule).
For ionic compounds of transition metals like copper, Roman numerals are used to show the type of ion in the compound. Therefore, CuO in option D should name as copper(II) oxide. This is because the copper ions in CuO exist as Cu2+.
[Note: Copper can form two types of ions, which are copper(I) ions (Cu+) or copper(II) ions (Cu2+)]
ZnO is correctly named as zinc oxide. Roman numeral is not needed here as all zinc ions exist as Zn2+.
Answer: B
Balancing ionic charges
People also ask
- Chemical Bonding and Compound Formation
- Chemical Bonding
- What is Covalent Bond?
- How is covalent bond is formed?
- Describe how to write a formula for a covalent compound
- What causes ions to form ionic bonds?
- Explain the formation of ionic bonds with examples
- Properties of Ionic and Covalent Compounds
- How do you write the formula for ionic compounds?
