What is the Molar Volume of a Gas at STP?
The Mole and the Volume of Gas
- It is rather tricky to find the number of moles of a gas by weighing its mass. Chemists determine the number of moles of any gas by measuring its volume. However, this cannot be done for solids and liquids.
- It is found that under the same temperature and pressure, equal volumes of all gases contain the same number of particles. Therefore, chemists introduced the concept of molar volume.
- Molar volume of a gas is defined as the volume of one mole of the gas.
- Thus, the molar volume is also the volume occupied by 6.02 x 1023 particles of gas.
- The molar volume of any gas is 22.4 dm3 mol-1 at STP or 24 dm3 mol-1 at room conditions.
Note: STP refers to standard temperature of 0°C and pressure of 1 atmosphere. Room conditions refer to the temperature of 25°C and the pressure of 1 atmosphere. - This means that one mole of any gas occupies the same volume at STP, which is 22.4 dm3. Under room conditions, one mole of any gas occupies 24 dm3.
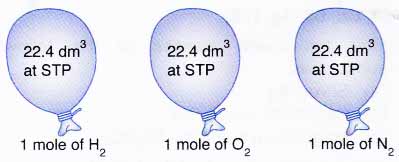 Figure above Each of these balloons contains 6.02 x 1023 gas molecules.
Figure above Each of these balloons contains 6.02 x 1023 gas molecules. - The following relationship shows how the volume of a gas can be converted to the number of moles and vice versa.

- In calculations, make sure that the volume of gas and the molar volume are of the same unit, that is, both are in cm3 or both are in dm3. Remember, 1 dm3 = 1000 cm3
People also ask
- What is the Relative Atomic Mass and Relative Molecular Mass of an Element?
- What is One Mole and How many Particles are in a Mole?
- How do you Calculate the Molar Mass of a Substance?
- How do you know the Order of Elements in a Chemical Formula
- What is Empirical and Molecular Formula?
- How do you Write a Chemical Equation?
The Mole and the Volume of Gas Problems with Solutions
1. What is the volume of 0.4 mole of carbon dioxide gas at STP?
[Molar volume: 22.4 dm3 mol-1 at STP]
Solution:
Given the number of moles of carbon dioxide, CO2 = 0.4 mol
Therefore, the volume of CO2 = number of moles of CO2 x molar volume at STP
= 0.4 x 22.4 dm3 = 8.96 dm3
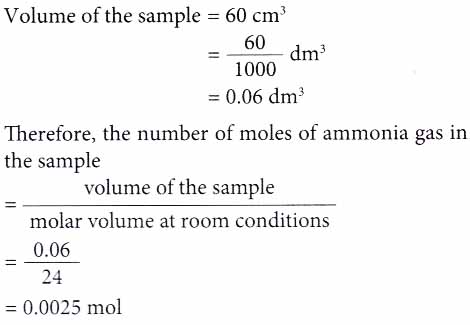
2. Find the number of moles of ammonia gas contained in a sample of 60 cm3 of the gas at room conditions. [Molar volume: 24 dm3 mol-1 at room conditions]
Solution:
The Relationship Between Mole, Number of particles, Mass and Volume
The following shows the relationships between the number of moles, number of particles, mass and volume of gases.
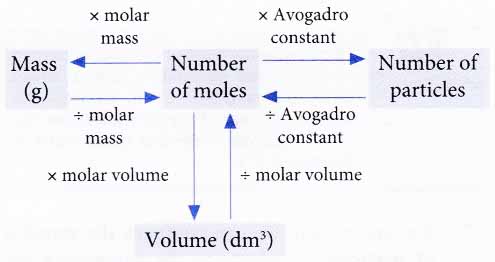
In most calculations, we first convert other quantities such as the number of particles, mass or volume to the number of moles (refer to Table).
Table: Summary of steps in calculations involving the number of moles
| Conversion | Steps |
| From mass to volume | Mass → number of moles → volume |
| From volume to mass | Volume → number of moles → mass |
| From volume to number of particles | Volume → number of moles → number of particles |
| From number of particles to volume | Number of particles → number of moles → volume |
The Relationship Between Mole, Number of particles, Mass and Volume Problems with Solutions
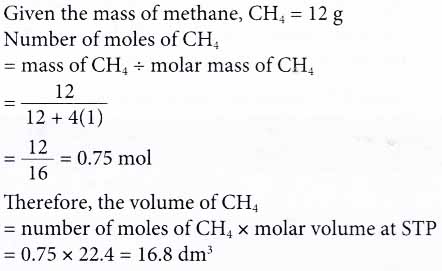
1. What is the volume of 12 g of methane at STP?
[Relative atomic mass: H, 1; C, 12. Molar volume: 22.4 dm3 mol-1 at STP]
Solution:
2. A sample of 120 cm3 of carbon dioxide is collected at room conditions in an experiment. Calculate the mass of the sample of carbon dioxide.
[Relative atomic mass: C, 12; O, 16. Molar volume: 24 dm3 mol-1 at room conditions]
Solution:
Given the volume of carbon dioxide, CO2 = 120 cm3 = 120/1000 dm3 = 0.12 dm3
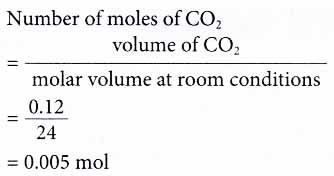
Therefore, the mass of CO2 = number of moles of CO2 × molar mass of CO2 = 0.005 × [12 + 2(16)]
= 0.005 × 44
= 0.22 g
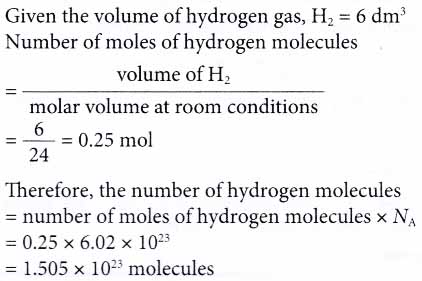
3. How many hydrogen molecules are there in 6 dm3 of hydrogen gas at room conditions? [Molar volume: 24 dm3 mol-1 at room conditions. Avogadro constant, NA: 6.02 × 1023 mol-1]
Solution:
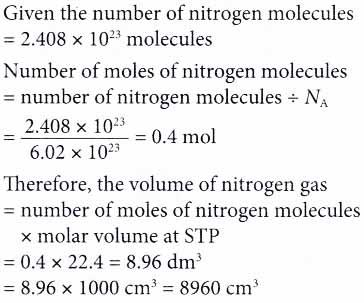
4. Find the volume of nitrogen gas in cm3 at STP that consists of 2.408 × 1023 nitrogen molecules. [Molar volume: 22.4 dm3 mol-1 at STP. Avogadro constant, Na: 6.02 × 1023 mol-1]
Solution: