Methods Of Separation Of Substances Under Wet Conditions
Some solid particles are insoluble in water. For example chalk powder, dust particles, sand, and tiny pieces of straw are insoluble in water. To separate this type of particles generally we use the following methods
Sedimentation and Decantation
Have you seen pulses being washed in your home? When pulses are kept in a bowl of water, they settle down as they are heavy. However, dirt, insects, tiny pieces of straw, and other lighter impurities float at the top. The water, which contains these impurities, is then poured out and discarded. This process involves two methods: sedimentation and decantation.
The process of separating insoluble solids, suspended in a liquid, by allowing them to settle down is called sedimentation. The solid particles that settle down during sedimentation are called sediments. The process of pouring out the clear upper liquid without disturbing the sediments is called decantation. The liquid above the sediments is called a supernatant.
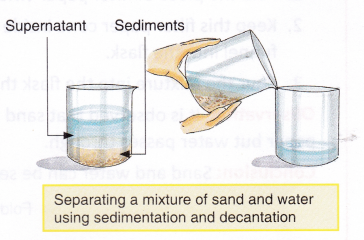 A mixture of sand and water can also be separated by sedimentation and decantation. The mixture is left undisturbed for some time. Sand, being heavier, settles down and water is poured out into a separate container.
A mixture of sand and water can also be separated by sedimentation and decantation. The mixture is left undisturbed for some time. Sand, being heavier, settles down and water is poured out into a separate container.
Activity Aim: To observe cleaning of rice by sedimentation and decantation.
Materials needed: A cup of rice and a bowl of water.
Method:
- Observe the rice and record your observations. Does the rice look dirty?
- Now, mix the rice with water in a bowl and allow it to stand for something.
- Without disturbing the layer of rice, decant the water.
Observation: When the mixture is allowed to stand, most impurities came up and floated near the surface, whereas the rice settled down. On decanting the water, we got cleaner rice.
Conclusion: Rice can be cleaned by sedimentation and decantation.
Filtration
The process by which two substances (an insoluble solid and a liquid) are separated by passing the mixture through a filtering device is called filtration.
Filtration is commonly used in our homes. For example, after preparing tea, we filter out the tea leaves using a strainer. Filtration is also done to remove pulp from fresh fruit juice. Water may also contain solid impurities, which can be removed by filtration.
During filtration, the insoluble solid is retained in the filtering device whereas the liquid passes through it. It is important that the particles of the insoluble solid are bigger than the holes in the filtering device for them to be retained in it. A filter paper is a filtering device that has very fine pores in it.
Activity
Aim: To separate a mixture of sand and water by filtration.
Materials needed: Mixture of sand and water, funnel, beaker, flask, and filter paper.
Method:
- Fold the piece of filter paper twice to make a cone.
- Keep this filter paper cone inside the funnel. Place the funnel into the flask.
- Pour the mixture into the flask through the funnel.
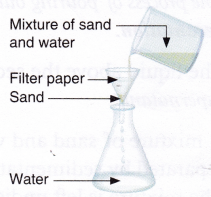
Observation: It is observed that sand is retained in the filter paper but water passes through.
Conclusion: Sand and water can be separated by filtration.
Evaporation
The process in which a liquid changes into a gas is called evaporation. In this method, the mixture is heated. The liquid part of the mixture evaporates leaving the solid part behind. For example, a mixture of common salt and water can be separated by evaporation. In fact, evaporating seawater is one of the oldest ways of obtaining salt.


Activity
Aim: To separate a mixture of salt and sand.
Materials needed: Mixture of salt and sand, filter paper, burner; test tube, test tube holder and water.
Method:
- Take the mixture of salt and sand in a test tube. Add water to it and shake it well.
- Salt dissolves in water, whereas sand does not. Sand can be separated using a filter paper.
- Now, only salt solution remains in the test tube.
- Heat the salt water till all the water changes to vapour, leaving the salt behind.
Observation: Salt is left behind in the test tube.
Conclusion: Two processes, filtration and evaporation, were used to separate a mixture of salt and sand. Thus, sometimes more than one method can be used to separate the components of a mixture.
Condensation
The process in which gas changes into liquid is called condensation. Condensation is the opposite of evaporation. In nature, water vapour in the air condenses to form its liquid form, the dew. Condensation takes place only when water vapour hits a cold surface.
Activity
Aim: To separate both water and salt from saltwater.
Materials needed: Saltwater, kettle, metal plate, a pair of tongs, bowl and burner.
Method:
- Take saltwater in the kettle and heat it over the burner.
- After some time, steam comes out of the spout of the kettle.
- Hold the metal plate (using tongs) above the spout. The steam condenses and changes into water droplets on touching the plate (cold surface).
- Collect the falling water drops in the bowl.
- Heat until all the water has boiled off.
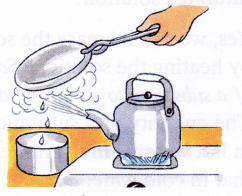
Observation: Salt is left behind in the kettle and water is collected in the bowl.
Conclusion: Water and salt could be separated from saltwater by the process of evaporation and condensation.
