ICSE Solutions for Class 10 Chemistry – Metallurgy
ICSE SolutionsSelina ICSE Solutions
APlusTopper.com provides ICSE Solutions for Class 10 Chemistry Chapter 7 Metallurgy for ICSE Board Examinations. We provide step by step Solutions for ICSE Chemistry Class 10 Solutions Pdf. You can download the Class 10 Chemistry ICSE Textbook Solutions with Free PDF download option.
Download Formulae Handbook For ICSE Class 9 and 10
Short Questions
Question 1: (i) Arrange Cu, Ca, Al, Fe, Mg, Pb, Na and Zn in the decreasing order, in which they appear in the activity series; putting down the most reactive metal first and least reactive in the last.
(ii) (a) Among the above metals, write the names of metals which will displace hydrogen from water or steam.
(b) Give two evidences to show that magnesium is more reactive than iron.
Answer: (i) The given metals are arranged in the activity series of metals as follows: Na, Ca, Mg, Al, Zn, Fe, Pb (most reactive) and Cu (least reactive)
(ii) (a) (1) Sodium and calcium displace hydrogen from cold water.
2Na + 2H2O ⟶ 2NaOH + H2 ↑
Ca + 2H2O ⟶ Ca(OH)2 + H2↑
(2) Magnesium and zinc metals are less reactive as they react with boiling water to liberate hydrogen gas.
Mg + 2H20 ⟶ Mg(OH)2 + H2↑
Zn + 2H20 ⟶ Zn(OH)2 + H2↑
(3) Iron which is less reactive, reacts in red hot conditions with steam to liberate hydrogen gas.
3Fe + 4H20 ⟶ Fe3O4 + 4H2↑
(4) Lead and copper almost fail to liberate hydrogen gas in any conditions, because they are not so reactive. They lie just above and below hydrogen in activity series of metals.
(b) (1) Magnesium reacts with boiling water to liberate hydrogen gas, while iron can do so with steam in red hot condition only.
(2) Magnesium can displace hydrogen from acids vigorously in cold but iron displaces hydrogen slowly.
Question 2: (i) Na, Ca, Mg, Al, Zn, Fe, Pb and Cu, are well known metals.
(a) X, Y and Z are coded letters for three of the metals in the activity series of metals as given above,
Metal X, reacts violently with cold water and its hydroxide is not decomposed by heat.
Metal Y, has no reaction with water but its hydroxide decomposes, with slight warming, giving a black powder.
Metal Z, reacts vigorously with dilute hydrochloric acid but hardly at all with cold water. If it is heated in steam, a white solid A is formed and a colourless gas B is set free
(1) which of the metals in the list is X ?
(2) which of the metals in the list is Y ?
(3) which of the metals in the list is Z ?
(4) Write the name of the solid A and gas B.
(b) State whether the following are soluble or insoluble in water.
(1) The carbonate of X.
(2) The carbonate of Y.
(3) The hydroxide of Z.
(ii) A certain metal does not liberate hydrogen from dilute sulphuric acid but it displaces silver from aqueous silver nitrate solution. State the most likely place for the metal in the activity series.
(iii) What would you expect to happen, if aluminium metal is heated with iron (III) oxide ? Also write the equation.
Answer: (i) (a) (1) The metal X is sodium.
(2) The metal Y is copper.
(3) The metal Z is magnesium.
(4) The name of the solid A is magnesium hydroxide, while the gas B is hydrogen.
(b) (1) Soluble as sodium carbonate is soluble in water.
(2) Insoluble, as copper carbonate is insoluble in water.
(3) Soluble, as magnesium hydroxide is soluble in water.
(ii) The metafiles below hydrogen and above silver in the activity series of metals.
(iii) When aluminium metal is heated with iron (III) oxide with metallic iron, an enormous amount of heat is produced due to the exothermic nature of the reaction. Molten iron is thus produced, which can be used in welding.
Fe2O3 + 2Al ⟶ Al2O3 + 2Fe + Q.
Question 3: (i) Arrange Ca, Pb, Fe, Na, Zn, Cu, and Al in the decreasing order of their reactivity.
(ii) Answer the following question related to above (i) sequence :
(a) Which of these is most likely to tarnish readily when exposed to the air ?
(b) Which of these is most likely to be found in free state in nature ?
(c) Which of these is most likely to react with cold water ?
Answer: (i) The decreasing order of the given metals is as follows :
[Most reactive] Na, Ca, Al, Zn, Fe, Pb, and Cu [Least reactive]
(ii) (a) Sodium [Na].
(b) Copper [Cu].
(c) Sodium [Na] and calcium [Ca].
Question 4: (i) From the metals copper, zinc, magnesium, sodium and iron, select the metal in each case which:
(a) Does not react with dil. hydrochloric acid.
(b) Has a hydroxide that reacts with both acids and alkalies.
(c) Does not react with cold water but reacts with steam when heated.
(d) Can form +2 and +3 ions.
(ii) Arrange the metals in decreasing order of reactivity.
Answer: (i) (a) Copper (b) Zinc
(c) Magnesium (d) Iron
(ii) Sodium > Magnesium > Iron > Zinc > Copper.
Question 5: (i) Differentiate between:
(a) Slag and Flux. (b) Calcination and Roasting.
(ii) Compare the properties of a typical metal and a non-metal on the basis of the following :
(a) Electronic configuration (b) Nature of the oxides
(c) Oxidising or reducing action (d) Conductivity of heat and electricity.
(iii) What are the differences between a mineral and an ore ?
Answer: (i) (a)
| Slag | Flux |
| It is the product obtained by the combination of the flux with gangue in metallurgy. | It is a substance which is added along with charge to separate the gangue in metallurgy. |
(b)
| Calcination | Roasting |
| It is the process of heating concentrated ore in a limited supply of air to a temperature insufficient to melt the ore. | It is the process of heating concentrated ore in a free supply of air to a temperature insufficient to melt the ore. |
| During calcination, no other chemical change occurs except decomposition. | During roasting, chemical changes like oxidation or reduction take place. |
(ii) (a) Metals complete their octet by the loss of electrons whereas non-metals complete their octet by the gain of electrons.
Metals generally contain 1 to 3 valence electrons in their outermost shell whereas non-metals contain 4 to 7 valence electrons in their outermost shell.
(b) Metals form basic oxides whereas non-metals form acidic oxides.
(c) Metals are reducing agents whereas non-metals act as oxidising agents.
(d) Metals He generally good conductors of heat and electricity whereas non-metals are bad conductors of heat and electricity.
(iii) (a) The minerals contain a low percentage of metal, while the ores contain a large percentage of the metal.
(b) The metal cannot be extracted from mineral, on the other hand ores can be used for the extraction of metal.
(c) All minerals are not ores, but all ores are minerals.
Question 6: (i) The ore zinc blende, is an important source of the metal zinc. What is the name of the zinc compound in zinc blende ?
(ii) What is the zinc compound obtained by roasting zinc blende ?
(iii) What is the type of chemical reaction carried out in order to obtain zinc ?
(iv) Are liquid zinc and liquid lead miscible or immiscible ?
(v) What is the name of the alloy formed between zinc and copper ?
Answer: (i) Zinc sulphide (ZnS).
(ii) Zinc blende is oxidized to zinc oxide by roasting in presence of excess air,
(iii) Reduction of zinc oxide.
(iv) Immiscible.
(v) Brass [7% of Cu, 30% of Zn].
Question 7: The following questions refer to the extraction of alumnium and iron from their ores :
(i) “Name the principal ore from which; (a) iron and (b) aluminium are extracted.
(ii) What is the most important chemical process in the extraction of any metal ? State how this essential step is carried out in the extraction of; (a) iron, (b) aluminium.
(iii) Iron and aluminium ores both, contain impurities. Explain briefly how these impurities are removed in each case.
(iv) What is the major impurity present in iron when it is removed from the blast furnace ?
Answer: (i) (a) Haematite (Fe2O3). (b) Bauxite (Al2O3).
(ii) Reduction of the oxide is an important step in extraction of metal.
In case of iron, Fe2O3 + 3CO ⟶ 2Fe + 3CO2
Al2O3 cannot be easily reduced, hence it is subjected to electrolysis. Aluminium is collected at the cathode.
(iii) Iron ore contains impurities of silica and sand. These are removed by magnetic separation. Bauxite and aluminium ore contains impurities of FeO and SiO2.
Bauxite containing FeO is calcinated at high temperature when FeO is oxidised to Fe2O3. Calcinated ore is then treated with NaOH when Al2O3 is converted into soluble NaAlO2.Fe2O3 can thus be filtered off. Bauxite containing SiO2 is mixed with coke and heated to 1000°C in an atmosphere of N2. Silica is reduced to Si which volatilises at the temperature of reaction.
Aluminium oxide is converted into AIN which is hydrolysed with water to obtain Al(OH)3.
(iv) Carbon is major impurity present in iron.
Question 8: (i) What is bauxite ? Which metal is extracted from it ?
(ii) In the electrolysis of molten alumina, the carbon anode is gradually burnt away. Why ?
(iii) Describe modem method of aluminium extraction.
Answer: (i) Bauxite is hydrated aluminium oxide [Al2O3.2H20] and aluminium metal is extracted from bauxite.
(ii) In the electrolysis of molten aluminium oxide, oxygen gas is liberated which gradually bums away carbon anode at a higher temperature to form carbon dioxide.
C + O2 ⟶ CO2
(iii) In the modern method, pure alumina is dissolved in cryolite [Na3.AlF6], which makes it good conductor of electricity.
When an electric current is passed through electrolyte, the heat is also produced which keeps the mass in molten state and alumina gets reduced to free aluminium metal according to the following reactions:
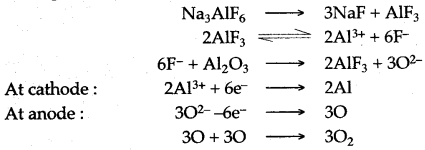
Question 9: The following questions are relevant to the extraction of Aluminium :
(i) State the reason for addition of caustic alkali to bauxite ore during purification of bauxite.
(ii) Give a balanced chemical equation for the above reaction.
(iii) Along with cryolite and alumina, another substance is added to the electrolyte mixture. Name the substance and give one reason for the addition.
Answer: (i) Caustic alkali dissolves aluminium oxide forming soluble sodium aluminate while impurities remains insoluble and ppt. as red mud.
(ii) Al2O3. 2H20 + NaOH ⟶ 2NaAlO2 + 3H2O
(iii) The name of substance is Fluorspar (CaF2) and it increases conductivity of the electrolyte.
Question 10: ‘Alumina (aluminium oxide) has a very high melting point of over 2,000°C so that it cannot readily be liquefied. However, conversion of alumina to aluminium and oxygen, by electrolysis, can occur when it is dissolved in some other substance.’
(i) Which solution is used to react with bauxite as a first step in obtaining pure aluminium oxide ?
(ii) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. Write the equation for this reaction.
(iii) Name the element which serves both as the anode and the cathode in the extraction of aluminium.
(iv) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis.
(v) Give the equation for the reaction which occurs at the anode when aluminium is purified by electrolysis.
Answer: (i) Sodium hydroxide
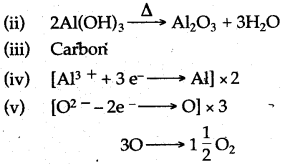
Question 11: (i) Give the name and formula of the ore of zinc containing its sulphide.
(ii) Write equations for the following steps in the extraction of zinc.
(1) Roasting of the ore.
(2) Reduction of the zinc compound which is the product of the above reaction.
Or
In the process of extracting zinc, the above named ore is roasted. Write the equation for the reaction which takes place when the sulphide ore is roasted. ”
(iii) What in addition to a zinc compound, is put into the blast furnace ?
(iv) State one large scale use of zinc.
(v) “Iron is removed from a blast furnace as a liquid”. State how zinc leaves a furnace.
Answer: (i) Zinc blende (ZnS)
(ii) (a) 2ZnS + 3O2 ⟶ 2ZnO + 2SO2
(b) ZnO + C ⟶ Zn + CO
(iii) Powdered coke.
(iv) For galvanising iron sheets to prevent rusting.
(v) Because of the maximum liberated heat coke reduces zinc oxide to zinc vapours to volatize a brilliant glow. These received in a condenser to liquifies, called spelter.
Question 12: (i) (a) What is the function of adding the lime stone in the “Blast Furnace”?
(b) Give two uses of slag.
(ii) How is zinc extracted from zinc blende [ZnS] ?
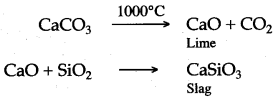
Answer: (i) (a) Lime stone decomposes at a higher temperature of blast furnace, to form calcium oxide and carbon dioxide.
CaCO3 ⟶ CaO + CO2 (Carbon dioxide)↑
Calcium oxide combines with sand, present as impurities, to form calcium silicate known as the slag.
CaO + Si02 ⟶ CaSiO3 (Calcium Silicate) [Slag]
The slag, is formed at the surface of blast furnace from where, it is removed from time to time. Thus, lime stone is used to remove the impurities of silicon dioxide present in ore.
(b) (1) It is used in the manufacture of cement.
(2) It is used as a fertilizer.
(ii) Zinc blende is strongly heated in the presence of aif in a ‘reverberatory furnace’, where it is oxidized to zinc oxide and sulphur dioxide gas is liberated.
2ZnS + 3O2 ⟶ 2ZnO (Zinc oxide) + 2SO2 ↑
Zinc oxide so obtained is mixed with carbon and is strongly heated, where it is reduced to metallic zinc and carbon monoxide are formed.
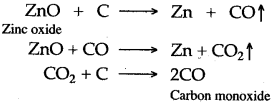
Carbon monooxide is regenerated.
Question 13: (i) The following is a list of metals : Na, Mg, Al, Zn, Fe, Pb, Cu and Au.
Name an ore of one of the above metals and state, how the metal is produced from it commercially by the process of:
(a) Electrolysis of the molten ore (b) Reduction by coke
(c) Roasting followed by reduction (d) Metal found in native state
(ii) How would you prove that the alloy solder contains lead ?
Answer: (i) (a) Aluminium-Bauxite. (b) Iron—Hematite.
(c) Lead—Galena. (d) Gold.
(ii) Take the given sample of solder and dissolve it in concentrated nitric acid. Dilute the solution with water and add dilute hydrochloric acid. A white precipitate of lead chloride is obtained, which is soluble in hot water. To this solution add potassium chromate solution, a golden yellow precipitate, confirms the presence of lead.
Pb + 4HNO3 ⟶ Pb(NO3)2 + 2H2O + 2NO2
Lead present in solder is confirmed as below :
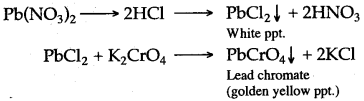
Question 14: The basic materials needed for production of iron in the blast furnace are lime stone, coke and air in addition to the iron ore.
(i) (a) Name one iron ore and write its formula.
(b) Hot air is blown in, at the base of the furnace; where’ it reacts with coke. Give the equation for the reaction that takes place.
(c) Higher up in the furnace, the iron ore is reduced to iron by one of the gases produced in the furnace. Give the chemical equation for the reaction, by which this gas is produced and give a balanced equation to show, how the ore is reduced to iron.
(ii) (a) Which compound produced from the limestone takes part in forming the slag ? Give the formula of the slag.
(b) What is |he principal use of slag ?
(iii) (a) Iron forms two series of slags ferrous and ferric. Using caustic soda solution, how would you distinguish between these two salts ?
(b) How zinc is extracted from calamine ore ?
Answer: (i) (a) Haematite (Ferric oxide) Fe2O3.
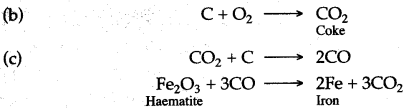
(ii) (a) The limestone decomposes at 1000°C, to form lime and carbon dioxide. Lime is the compound which takes part in the formation of slag.
Slag is calcium silicate with the formula CaSiO3.
(b) The slag is used in the manufacture of cement.
(iii) (a) When caustic soda solution is added to a ferrous salt, a dirty green precipitate of ferrous hydroxide is obtained.
![]()
On the other hand, ferric salts give a reddish brown precipitate of ferric hydroxide with caustic soda.
![]()
(b) Calamine ore is chemically zinc carbonate [ZnCO3]. Purified ore is roasted in a reverberatory furnace, where it is decomposed to form zinc oxide and carbon dioxide gas is liberated.
ZnCO3 ⟶ ZnO + CO2
Zinc oxide so obtained is mixed with carbon and heated in a specially designed clay retorts, placed in a furnace, where it is reduced to zinc metal and carbon monoxide is formed, which is further used to reduce the ore.
ZnO + C ⟶ Zn + CO
ZnO + CO ⟶ Zn + CO2
Question 15: (i) (a) Give the common name of an ore, from which aluminium is extracted.
(b) Name the process of its extraction and describe it.
(c) Name another metal, which is extracted by this process.
(ii) (a) Give a reason, why aluminium cannot be obtained from aluminium oxide; by the “Blast Furnace Process” or “Carbon Reduction Process.”
(b) Name the properties for which aluminium is used in:
(I) Cooking utensils, (II) Overhead electric transmission wire.
(c) Aluminium is more active than iron and yet there is less corrosion of the aluminium, when both are exposed to air. Explain this fact.
(iii) (a) What is pig-iron ?
(b) What is the main difference in chemical composition of cast iron and steel ?
Answer: (i) (a) Aluminium metal is extracted from Bauxite ore [Al2O3. 2H2O].
(b) Aluminium metal is extracted by electrolytic reduction process, in which electric current is passed through fused aluminium oxide, the purified Bauxite. Aluminium metal is collected at cathode, while oxygen gas is liberated at anode according to the following reactions.
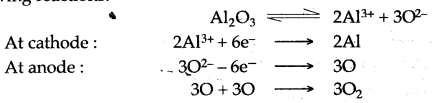
(c) Calcium metal is also extracted by electrolytic reduction process.
(ii) (a) Aluminium metal is more electropositive than iron. It has the characteristic property of all the oxides of metals that highly electropositive oxides of metals cannot be reduced by heating with carbon to form free metal. Hence aluminium is not extracted by the “Blast Furnace Process”.
(b) (I) Aluminium is a good conductor of heat, light in weight, strong and can be made passive by nitric acid.
(II) Aluminium is a good conductor of electricity, light in weight, tough and possess high tensile strength and is resistant to corrosion. It is a cheaper metal than copper.
(c) Aluminium metal is protected by a thin film of aluminium oxide, which sticks firmly with the metal and prevents further corrosion.
(iii) (a) The iron, which is obtained directly from the blast furnace is known as pig iron. Besides iron, it contains about 4% of carbon and small quantities of silicon, phosphorus, manganese and sulphur. When pig iron is heated to melting and is casted into desired moulds, then it is known as cast iron.
(b) Cast iron contains 2-5% to 4% of carbon, while steel contains 0-5% to 1-5% of carbon. Due to the higher percentage of carbon in cast iron, it resists corrosion.
Question 16: (i) What is froath floatation process and for, what purpose it is used ?
(ii) How is the metal sodium extracted ? Write the equations for the reactions involved.
(iii) Name two other metals, which can be extracted by electrolytic reduction method.
Answer: (i) In this process, the heavy material containing metal, is floated upward with froath to separate it from,waste material present in ore or mineral. Hence it is called froath floatation process.
(ii) Sodium metal is Extracted by the electrolysis of fused sodium chloride. Sodium is collected at cathode, while chlorine gas is liberated at anode; as an important by product.
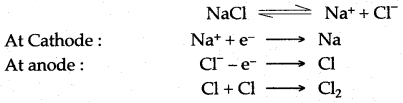
(iii) Calcium, and magnesium are other two metals, which can be extracted by electrolytic reduction method.
Question 17: (i) Give the reactions taking place in the blast furnace, when haematite, coke and lime stone are added to it.
(ii) What is the purpose of adding lime stone in the extraction of iron from haematite ?
(iii) What is the use of galvanized iron ?
(iv) Which two products are taken out from the blast furnace during the extraction of iron ?
Answer: (i) (a) Carbon burns in the presence of oxygen of air to form carbon dioxide which is reduced to carbon monoxide in the presence of coke.
C + O2 ⟶ CO2
CO2 + C ⟶ 2CO
(b) Carbon monoxide so produced, reduces haematite ore [Ferric oxide] to metallic iron.
Fe2O3 + 3CO » 2Fe + 3CO2↑
(c) Lime stone, at a higher temperature of blast furnace; decomposes into calcium oxide and carbon dioxide.
CaCO3 ⟶ CaO + CO2↑
(d) Calcium oxide so formed reacts with sand [silicon dioxide] to form calcium silicate, the slag and thus the impurity of sand is removed.
CaO + SiO2 ⟶ CaSiO3
(ii) Lime stone is added to “blast furnace” in the extraction of iron to remove the impurities of silicon dioxide, i.e., the sand.
(iii) Galvanized iron is used in making different varieties of tools for industries, scientific apparatus, and household fittings.
(iv) Pig iron and slag are the two products, which are taken out from the blast furnace during the extraction of iron.
Question 18: (i) The following represents a summary of the reaction which occur in the “Blast furnace”, leading to the production of molten iron.
Coke + O2 ⟶ Gas Y
Gas Y + Coke ⟶ Gas Z
Gas Z + Iron oxide ⟶ Gas Y + Iron
Identify the gases, Y and Z. Explain why gas Z is said to act as a reducing agent in the last step in the above equations.
(ii) Calcium, Copper, Lead, Aluminium, Zinc, Chromium, Magnesium, Iron,.
Choose the major metals from the list given above to make the following alloys:
(a) Stainless steel. (b) Brass.
Answer: (i) The gas Y, carbon dioxide and the gas Z, is carbon monoxide. The gas Z is a reducing agent because it removes oxygen from iron oxide and converts it into metallic iron and itself gets oxidized to carbon dioxide.
(ii) (a) Iron, Chromium, (b) Zinc.
Question 19: (i) (a) Name two ores of iron.
(b) Name three raw materials used in the extraction of iron.
(c) Write equations that occur in the “Blast Furnace”.
(ii) What are the main constituents of steel ?
(iii) What is tempering of steel ?
Answer: (i) (a) Two main ores of iron are : Haematite [Fe2O3] and Magnetite [Fe2O4].
(b) Iron ore, lime stone, and coke are used in the extraction of iron.
(c) Following are the reactions, which take place in the “Blast Furnace”, during the extraction of iron.
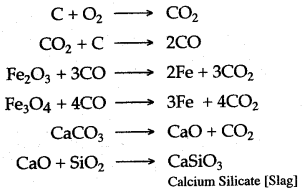
(ii) Steel is an alloy of iron and carbon containing very small amounts of impurities, that are present in the cast iron. The carbon content varies from 0-5 to 1-5%. The variety containing lower percentage of carbon is called “mild steel and the variety which contains higher percentage of carbon is known as “hard steel”.
(iii) The hardened steel is brittle in nature and when it is heated upto a definite temperature and for certain time and then allowed to be cooled down slowly, then it loses its brittleness. This process is known as tempering of steel and is employed for bringing the steel into a suitable state of hardness and elasticity. The temperature required is generally judged from the colour of a thin film of oxide which is formed on the surface and varies from yellow to brown to blue as the temperature rises from 200°C to 300°C.
Question 20: (i) Most of pig iron obtained from blast furnace is converted into steel. Suggest a reason for this.
(ii) The element X, has the electronic configuration 2, 8, 8, 1. Describe the symbol for an ion of X. Give a reason fdr your answer and deduce whether the element X would be expected to have oxidizing or reducing properties.
(iii) How is the metal X, extracted from its given salt ?
Answer: (i) Pig-iron does not possess a high tensile strength, hence it cannot withstand with high stress and strain. Pig-iron is brittle in nature and cannot be welded. Due to these disadvantages, pig-iron cannot be put in different varieties of uses hence it is converted into steel.
(ii) The element X, contains one electron in the outermost orbit of its atom, which can be easily donated and one unit of positive charge is gained on its ion.
X – e– ⟶ X+
The symbol of X ion is X+. The atom of element X is a donor of electron hence X is expected to have reducing properties.
(iii) It is clear from the properties of X, that the metal X is supposed to be highly positive in nature, hence it is extracted by electrolytic reduction method.
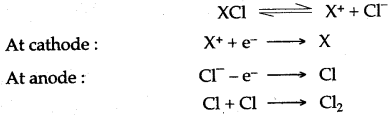
Question 21: A to F below relate to the source and extraction of either Zinc or Aluminium.
A. Bauxite: B. Coke
C. Cryolite D. Froth floatation
E. Sodium hydroxide solution. F. Zinc blende.
(i) Write down the three letters each from the above list which are relevant to :
(1) Zinc (2) Aluminium.
(ii) (1) Metals are generally solid at room temperature. Name the metal which is liquid at room temperature [say 25°C].
(2) Which allotrope of the non-metal conducts electricity ?
(iii) How many valence electrons are present in (a) Metals, (b) Non-metals,
Answer: (i) (1) B, D, F (2) A, C, E
(ii) (1) Mercury metal exists in liquid state at room temperature.
(2) Graphite, an allotrope of carbon is a good conductor of electric current.
(iii) Atom of metals contain 1, 2 and 3 valence electrons, while the atom of non-metals contain 4,5, 6 and 7 valence electrons.
Question 22: The following substances are put into the blast furnace while manufacturing iron :
Iron ore, coke, limestone and hot air. In this context—
(i) What is the name of the most common ore of iron and what is its chemical formula ?
(ii) What is the purpose of using :
(a) The coke (b) The limestone
(iii) Write the equation for the reduction reaction which produces iron.
(iv) Name the substance which is collected along with cast iron at the bottom of the furnace.
(v) Write the chemical equation for the formation of the substance named in (iv) above.
Answer: (i) Haematite—Fe2O3.
(ii) (a) Coke acts as a reducing agent, (b) Limestone acts as a flux.
(iii) Fe2O3 + 3CO ⟶ 2Fe(Spongy iron) + 3CO2
(iv) Fusible slag (calcium silicate)
(v) CaO + SiO2 ⟶ CaSiO3
Question 23: (i) Name an alloy used in aircraft construction and give a reason for its use.
(ii) What is rusting of iron ?
(iii) (a) How are the following protected from rust ?
(1) A car bumper and (2) A food can.
(b) How can iron or steel be prevented from rusting, when used for ?
(c) What is galvanized iron and for what purposes it is used ?
(d) To protect iron from rusting it is coated with a thin layer of zinc. Name this process.
Answer: (i) Duralumin, an alloy of aluminium, is used in the construction of aircraft; because it is light, resistant to corrosion and has great tensile strength.
(ii) The rusting of iron is a process of atmospheric corrosion, i.e., slow destruction of iron by moisture and atmospheric oxygen. Rust is a reddish-brown powdery deposit and consists of a mixture of ferric hydroxide and hydrated ferric oxide.
(iii) (a) (1) Nickel Plating and (2) Galvanizing.
(b) By painting and by coating with nickel.
(c) Iron coated with zinc is called galvanized iron. Galvanization is a process of depositing a thin layer of zinc, over the surface of iron to protect iron from rusting. Zinc is more electropositive and would be attacked first and thus iron is protected from any corrosion. Galvanized iron is used in making different varieties of tools for industries, scientific apparatus and household fittings.
(d) Galvanisation.
Question 24: (i) The ore zinc blende, is an important source of the metal zinc. What is the name of the zinc compound in zinc blende ?
(ii) Which is the zinc compound obtained by roasting zinc blende ?
(iii) What is the type of chemical reaction carried out after roasting in order to obtain zinc ?
(iv) Are liquid zinc and liquid lead miscible or immiscible ?
(v) What is the name of the alloy formed between zinc and copper ?
Answer: (i) Zinc sulphide [ZnS].
(ii) Zinc blende is oxidised to zinc oxide by roasting in presence of excess air on reverberatory furance.
(iii) Reduction of zinc oxide.
(iv) Immiscible.
(v) Brass.
Question 25: (i) (a) With reference to the reduction of copper oxide, iron (II) oxide, lead (II) oxide and magnesium oxide by hydrogen; place the oxides in increasing order of reduction, i.e., first the oxide that is most difficult to reduce; and at last, the oxide that is most easy to reduced.
(b) (1) What is the type of bonding expected in metallic chloride ?
(2) If fused metallic chloride is electrolysed, at which electrode the metal will be obtained.
(3) ‘What metallic property is shown by the non-metal graphite ?
(c) (1) Cast iron contains about 4% of carbon. By which chemical process is the amount of carbon decreased to make steel ?
(2) Which metal is added to steel to make stainless steel ?
(ii) (a) For each substance listed below, explain its significance in the extraction of Aluminium:
(1) Bauxite. (2) Cryolite.
(3) Graphite. (4) Sodium hydroxide.
(b) The following questions relate to the extraction of aluminium by electrolysis :
(1) Give the equation for the reaction that takes place at the cathode.
(2) Explain why it is necessary to renew the anode from time to time.
Answer: (i) (a) The increasing order of oxides is :
Magnesium oxide > iron (II) oxide > lead (II) oxide > copper oxide.
The magnesium oxide is highly stable. It cannot be reduced by hydrogen, while the last three members are reduced by hydrogen to their metals according to reactivity series.
(b) (1) Electrovalent or ionic bond.
(2) Cathode.
(3) Non-metal graphite is good conductor of heat and electricity.
(c) (1) In the Bessemer process it takes only a few minutes to convert cast iron into steel.
(2) Stainless steel is an alloy which contains about 18% of Cr, 8% Ni and 1% C.
(ii) (a) (1) Bauxite : It is the main ore of aluminium from which aluminium metal can easily be extracted.
(2) Cryolite : It is added to lower the fusion temperature of the electrolytic bath. The mixture melts at 950°C instead of 2050°C thereby saving electrical energy. It also increases conductivity alongwith fluorspar.
(3) Graphite acts as an anode. Here the anode gets oxidised to carbon dioxide, i.e.
C + O2 ⟶ CO2
Or 2O2- – 4e– ⟶ O2
Thus, electrodes are made of graphite.
(4) Sodium hydroxide when added to powdered bauxite and the mixture when heated under pressure for 2-3 hours, bauxite is converted to soluble sodium aluminate (NaAlO2).
![]()
This solution is used, to obtain pure aluminium.
(b) (1) The following reaction (reduction) takes place at the cathode during the extraction of aluminium.
Al+3 + 3e– ⟶ Al.
(2) Oxygen gas is produced at the graphite anode, which combines with carbon to form carbon dioxide gas at high temperature and thus anode destroys away. Thus, it is necessary to renew the anode to continue the process of extraction of aluminium.
Question 26: (i) How are the alloys classified ?
(ii) What are ferrous alloys ? Give one example.
(iii) What are non-ferrous alloys ? Give one example.
(iv) An alloy usually has some property which makes it particularly useful. What is the special property of: (a) Type metal, (b) Duralumin ?
Answer: (i) Alloys are classified on the basis of their constituents. They are classified as follows:
(a) Ferrous alloys. (b) Amalgams. (c) Non-ferrous alloys.
(ii) Ferrous alloys: It is an alloy having iron as one of the constituent, e.g., nickel, steel.
(iii) Non-ferrous alloys: An alloy that does not contain iron as one of its constituents, is called a non-ferrous alloy, e.g., brass.
(iv) (a) Type metal is hard and expands on cooling and is therefore used for making types.
(b) Duralumin, is light and strong therefore it is used in the construction of air-craft.
Question 27: State the main components of the following alloys :
(i) Brass. (ii) Duralumin. (iii) Bronze. (iv) Stainless steel.
Answer: (i) Copper and zinc. (ii) Aluminium and magnesium.
(iii) Copper and Tin. (iv) Iron.
Question 28: Give the composition and uses of the following alloys :
(i) Brass. (ii) Bronze. (iii) German silver.
(iv) Type metal. (v) Magnalium (vi) Duralumin.
Answer: (i) It is an alloy of copper and zinc and is used for making utensils, condenser tubes, statues, and for making decoration pieces.
(ii) It is an alloy of copper, zinc and tin and is used for making statues, utensils and coins.
(iii) It is an alloy of copper, zinc and nickel and is used for making ornaments and utensils and also used for decoration.
(iv) It is an a Hoy of lead, antimony and tin and is used for making printing type.
(v) It is an alloy of aluminum and magnesium and is used for making light instruments, parts of machines and balance beams.
(vi) It is an alloy of aluminium, copper, magnesium and manganese and is used for making aeroplanes, space crafts, sea ship and pressure cookers.
Question 29: (i) Write a note on thermite welding.
(ii) What is meant by electrochemical series of metal ?
Answer: (i) Thermite is a mixture of 3 parts of ferric oxide and one part of aluminium powder. On the top of the thermite mixture, an irgnition mixture of potassium chlorate and magnesium powder is placed. A burning Mg riboon is inserted into the ignition mixture, which catches the fire and ignite the thermite. During this reaction ferric oxide is converted into iron and a large amount of heal is evolved and about 3000°C temperature is achieved.
Fe2O, + 2Al ⟶ 2Al2O3 + 2Fe + Heat
The formed liquid iron is allowed to drop over the gap between the broken piece and thus they join together.
This process is termed as boldschmidts aluminothermic process.
(ii) The arrangement of metals in the order of their decreasing activity, in which the most reactive at the top and the least reactive at the bottom are placed in the series. This arrangement of metals in a series is called electrochemical or metal activity series.
Question 30: (i) (a) How will you show that sodium is a metal ?
(b) How will you show that sulphur is a non-metal ?
(ii) (a) Which gas is liberated when aluminium metal reacts with a solution of sodium hydr¬oxide ?
(b) Which gas is generally liberated when metals react with dilute acid?
Answer: (i) (a) Sodium metal can form positive ions by the loss of one electron, this metal is electropositive.
Na – e– ⟶ Na+
Sodium has high density and is less dense then water.
(b) Sulphur is non metal because, it gives negative sulphur ions by gaining of two electrons. It dissolves in many liquid solvents, but it is non-conductor of electricity and heat.
S + 2e– ⟶ S2
(ii) (a) When aluminium metal reacts with sodium hydroxide solution, hydrogen gas is liberated.
(b) Hydrogen gas is generally liberated when metals react with dilute acid.
Figure/Table Based Questions
Question 1: The given sketch of an electrolytic cell used in the extraction of aluminium:
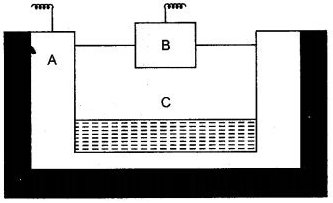
(i) What is the substance of which the electrode A and B are made ?
(ii) At which electrode (A ro B) is the aluminium formed ?
(iii) What are the two aluminium compound in the electrolyte C ?
(iv) Why is it necessary for electrode B to be continuously replaced ?
Answer: (i) Carbon (Graphite)
(ii) A
(iii) Aluminium oxide / Alumina / cryolite (sodium aluminium fluoride).
(iv) Burns away in the presence of oxygen produced or consumed.
Question 2: The given figure illustrates the refining of aluminium by Hoope’s process.
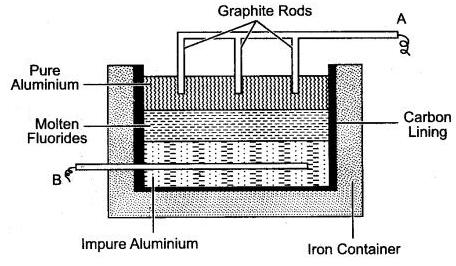
(i) Which of A and B is the cathode and which one is the anode ?
(ii) What is the electrolyte in the tank ?
(iii) What material is used for the cathode ?
Answer: (i) A—Cathode, B—Anode.
(ii) Mixture of fluorides.
(iii) Graphite
Question 3: Study the given figure and answer the following questions :
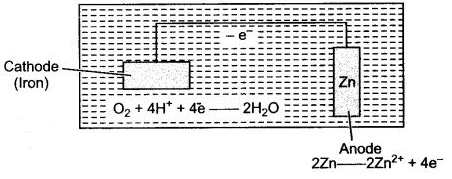
(i) What are the elements (any two) of which the electrodes are made ?
(ii) Name the process shown.
(iii) Explain the process and write the reactions occur an anode 4 cathode.
(iv) Where this method is used ?
Answer: (i) A is cathode made up of iron and B is anode made up of zinc or magnesium.
(ii) The process shown is cathode protection.
(iii) The iron cathode is to saved by connecting it, to a piece fo more electro-positive metal (anode). The electrons from anode get oxidised and move towards the cathode, cover it.
(iv) This method is used for protecting iron from rusting, e.g. underground sewer pipes and storage tanks.
Question 4: (i) Name the process and the element extracted by the above process as shown in the figure.
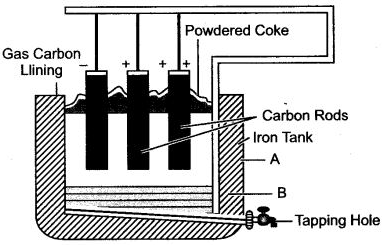
(ii) Give the function of three components of electrolyte.
(iii) Why is electrolyte covered with coke?
(iv) Write the electrolytic reaction taking place at cathode?
Answer: (i) Aluminium, Hall and Herault’s process.
(ii) Alumina (Al2O3) is the main aluminium yielding compound.
Cryolite [Na3AlF6] acts as a solvent and lowers the fusion temperature from 2050°C to 950°C.
Fluorspar (CaF2) acts as a solvent and increases the conductivity of electrolytic mixture.
(iii) A layer of powdered cpke is sprinkled over the surface of the electrolyte mixture because it reduces the heat loss by radiation and prevents carbon anode from brning in air.
(iv) ![]()
Aluminium formed sinks to the bottom of the tank and is periodically tapped off.
Question 5: List 1 contains the metals/alloys, (i), (ii), (iii), (iv), (v) and list 2 contains their uses A, B, C, D, E.
| List 1 | List 2 |
| (i) aluminium | A. steel making |
| (ii) lead | B. aeroplane wings |
| (iii) brass | C. galvanizing |
| (iv) iron | D. radiation shield |
| (v) zinc | E. electrical fittings |
Copy and complete the following table writing down the letter for the correct use of each metal. An answer may be used only once. The first has been done for you.
| Metal | (i) | (ii) | (iii) | (iv) | (v) |
| Use | B |
Answer:
| Metal | (i) | (ii) | (iii) | (iv) | (v) |
| Use | B | D | E | A | C |
Question 6: The table below compare some properties of metals and non-metals. Write down the missing statements (i) to (iv).

Answer: (i) Metals are good conductor of heat.
(ii) Non-metals are non-malleable [Brittle].
(iii) Non-metals form negative ions [Anions].
(iv) Metals form basic oxides.
Reasoning Based Questions
Question 1: Why are metals called reducing agents ?
Answer: They tend to lose electrons and act as reducing agents.
Question 2: Why are non-metals called oxidizing agents ?
Answer: They tend to gain electrons and act as oxidising agents.
Question 3: Why iron is not found in free state in nature ?
Answer: Iron is quite reactive metal, it easily combines with other metals. Iron thus occurs in nature in the form of its compounds and not as a free element.
Question 4: Iron liberates hydrogen from dilute sulphuric acid, while silver cannot. Why ?
Answer: In activity series of metal, iron occupies a higher position than hydrogen; while silver is placed below hydrogen; hence iron is more reactive than silver and is able to displace hydrogen from dilute sulphuric acid.
Fe + H2SO4 ⟶ FeSO4 + H2↑
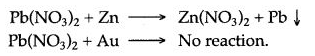
Question 5: Zinc displaces lead from lead nitrate solution, while gold is unable to do so. Why ?
Answer: Zinc is above lead in the metal activity series. It is more reactive than lead while gold, a noble metal, lies far below lead in the activity series and it is less reactive or highly unreactive. Zinc reacts with lead nitrate solution to precipitate lead and zinc nitrate is formed. There is no reaction between gold and lead nitrate.
Question 6: Why is sodium metal always stored under kerosene oil ?
Answer: Sodium is a very reactive metal and on exposure to moist air, the surface of sodium metal is tarnished due to formation of sodium carbonate.
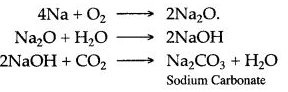
To avoid this sodium is always kept under kerosene oil.
Question 7: Why carbon can reduce copper (II) oxide to copper but not calcium oxide to calcium ?
Answer: Because carbon has greater affinity for oxygen than copper and less affinity for oxygen than calcium.
Question 8: Aluminium is highly electropositive metal, in spite of it aluminium does not oxidise rapidly in air. Why ?
Answer: In moist air, a thin layer of aluminium oxide is formed on it quickly which protects aluminium to oxidise. This is the reason why aluminium does not oxidise rapidly in air.
Question 9: Why extraction of aluminium is difficult ?
Answer: Extraction of aluminium is difficult because :
(i) Pure aluminium oxide melts at 2050°C only. So, a large amount of energy is needed to maintain this high temperature.
(ii) A good amount «f the aluminium vaporises at this temperature.
(iii) Fused alumina does not conduct electricity well.
Question 10: During the extraction of aluminium, cryolite and fluorspar are added to alumina. Why ?
Answer: Cryolite and fluorspar are added to alumina :
(i) To lower the melting point of aluminium.
(ii) To make alumina a good conductor of electricity.
(iii) Cryolite acts as a solvent for alumina.
Question 11: Aluminium transmission wires are preferred to copper transmission wires. Why ?
Answer: (i) Aluminium is lighter than copper.
(ii) It is a good conductor of electricity.
(iii) Aluminium is cheaper than copper.
Question 12: Why in construction work alloy duralumin is used rather than aluminium ?
Answer: Because duralumin is harder, stronger and more resistant to corrosion.
Balancing/Writing the Chemical Equations
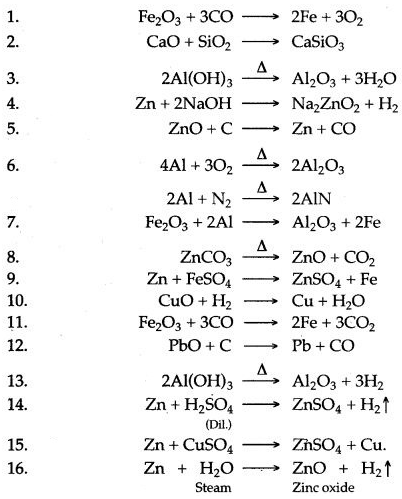
Question 1: Write balanced chemical equation:
1. The reduction of metallic oxide inside the blast furnace.
2. Formation of Hag inside the blast furnace.
3. Heating of aluminium hydroxide.
4. Reaction of zinc with hot concentrated sodium hydroxide.
5. Reduction of zinc oxide.
6. Burning of aluminium in air.
7. Reduction of ferric oxide by aluminium powder.
8. Calamine is heated.
9. Zinc placed in ferrous sulphate solution.
10. Reduction of copper oxide by hydrogen.
11. Reduction of iron (III) oxide by carbon monoxide.
12. Reduction of lead (II) oxide by carbon.
13. Action of heat on aliuninium hydroxide.
14. Zinc is treated with dilute sulphuric acid.
15. Action of Copper sulphate solution on zinc.
16. Action of Steam on Zinc.
Answer:
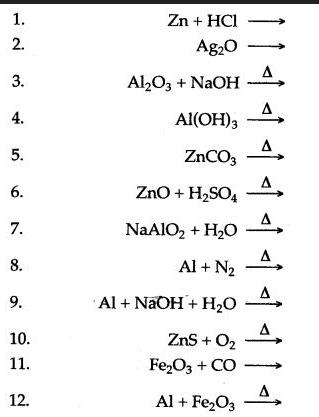
Question 2: Complete and balance the following equations :
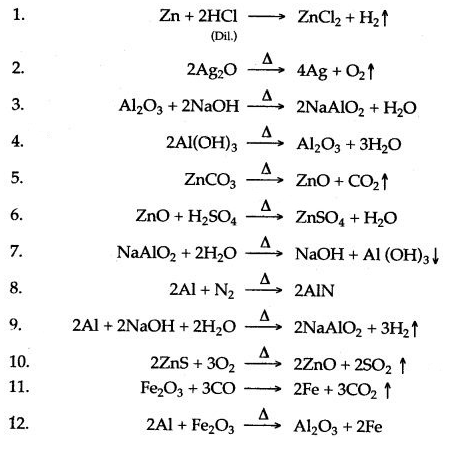
Answer:
IUPAC Naming/Writing the Structural Formula
Question : Give the chemical formulae of the following naturally occuring ores:
1. Cryolite
2. Galena
3. Corundum
4. Dolomite
5. Zincite
6. Malachite
7. Cinnabar
8. Gypsum
9. Horn silver
10. Epsom salt
11. Haematite
12. Bauxite
Answer:
1. Na3AlF6
2. PbS
3. AI2O3
4. CaCO3.MgCO3
5. ZnO
6. CuCO3.Cu(OH)2
7. HgS
8. CaSO4.2H2O
9. AgCl
10. MgSO4. 7H2O
11. Fe2O3
12. Al2O3.2H2O
For More Resources
