How did Mendeleev Arrange the Periodic Table
Mendeleev’s periodic Law and Mendeleev’s periodic table
While working systematically on the physical and chemical properties of elements, Dmitri Invanovich Mendeleev noticed that properties of elements varied regularly with the atomic mass. He arranged the 63 elements then known in a table on the basis of similarities in properties. It was found that most of the elements occupied places in the table in order of their increasing atomic masses. In 1869, Mendeleeve formulated a law, now known as the periodic law. The law is stated as follows.
The properties of elements are periodic functions of their atomic masses. This means, if the elements are arranged in order of increasing atomic masses then those with similar properties are repeated at regular intervals.
On the basis of the periodic law, Mendeleev presented his classification in the form of a table, now known as Mendeleev’s periodic table. A simplified version of this periodic table is given below. In this table, copper, silver and gold find places in groups I as well as VIII.
Groups → Periods ↓ | I | II | III | IV | V | VI | VII | VIII |
| 1 | H 1 | |||||||
2 | Li 7 | Be 9.4 | B 11 | C 12 | N 14 | O 16 | F 19 | |
| 3 | Na 23 | Mg 24 | Al 27.3 | Si 28 | P 31 | S 32 | Cl 35.5 | |
4 | K 39 | Ca 40 | ? | Ti 48 | V 51 | Cr 52 | Mn 55 | Fr Co Ni Cu 56 59 59 63 |
| 5 | Cu 63 | Zn 65 | ? 68 | ? 72 | As 75 | Se 78 | Br 80 | |
6 | Rb 85 | Sr 87 | Yt 88 | Zr 90 | Nb 94 | Mo 96 | ? 100 | Ru Rh Pd Ag 104 104 106 108 |
7 | Ag 108 | Cd 112 | In 113 | Sn 118 | Sb 122 | Te 125 | I 127 | |
8 | Cs 133 | Ba 137 | Di 138 | Ce 140 | ? | ? | ? | ? |
9 | ? | ? | ? | ? | ? | ? | ? | |
10 | ? | ? | Er 178 | La 180 | Ta 182 | W 184 | ? | Os Ir Pt Au 195 197 198 199 |
11 | Au 199 | Hg 200 | Tl 204 | Pb 207 | Bi 208 | ? | ? | |
12 | ? | ? | ? | Th 231 | ? | U 240 |
This table consists of vertical columns called groups and horizontal rows called periods. There are only eight groups in the table. Mendeleev left some vacant places (shown by question marks) for the yet undiscovered elements. Noble gases were not discovered then. So, he did not provide any place for them in his periodic table.
Mandeleev’s idea was remarkable in that he used a fundamental atomic property (atomic mass) as the basis of classification. While classifying elements he laid special emphasis on tow factors.
1. Similar elements were grouped together.
2. Elements were arranged in order of increasing atomic masses.
People also ask
- What is the periodic table of the elements?
- Physical and Chemical Properties of Group 1 Elements
- Physical and Chemical Properties of Group 18 Elements
- Physical and Chemical Properties of Group 17 Elements
- What do you mean by transition metals?
- What is the Need for Classification of Elements?
- Modern Periodic Table and Its Significance
- Periodic Trends in Properties of Elements
- What is Valency and Atomic Radius?
- What are Metallic and Nonmetallic Properties?
Modified version of Mendeleev’s Periodic Table :
The elements which were undiscovered and for whom Mendeleev had left vacant places were discovered later. Some of these are scandium (Sc), gallium (Ga) and germanium (Ge). These elements were accommodated in their proper places in the table. The elements helium (He), neon (Ne), argon (Ar), Krypton (Kr), Xenon (Xe) and radon (Rn) became known only towards the end of the nineteenth century. These elements, called noble gases, were placed in the table as a separate group, called 0 group. The periodic table had to be modified then. The modified version of the table is shown below.
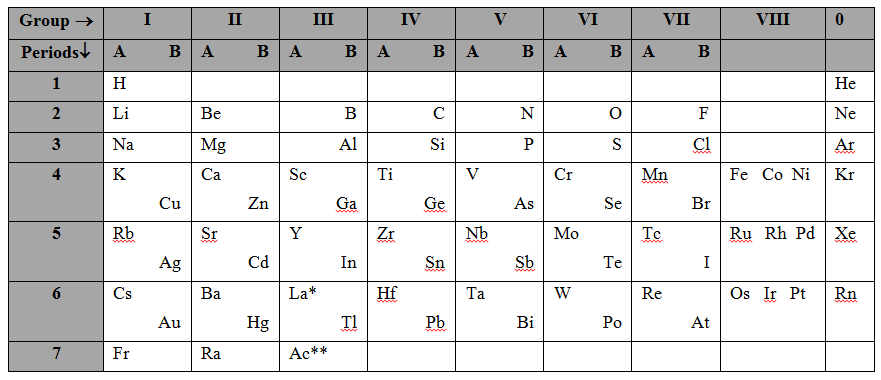
| Lanthanide series* (along with langhanum) | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
| Actinide series**(along with actinium) | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr |
Features of the modified version of Mendeleev’s periodic table :
1. Groups into subgroups : Each group of this periodic table is further divided into two subgroups A and B. The properties of elements within a subgroup resemble more markedly but they differ from those of the elements of the other subgroups. For example., lithium (Li), sodium (Na), potassium (K), etc., of subgroups IA have close resemblance of properties but they have hardly any resemblance to the coinage metals (Cu, Ag and Au) of subgroup IB.
Mendeleev allowed the subgroups to be represented within the same group.
2. Prediction of errors : This periodic table could predict errors in the atomic masses of some elements on the basis of their position in the periodic table. For example, when the periodic table was published, the experimental value of the atomic mass of beryllium (Be) we was supposed to be 13.65 and its valency, 3. So, the position of Be should have been somewhere else, but Mendeleev placed it at its appropriate position on the basis of its properties. He further suggested that the atomic mass of Be needed correction. Mendeleev predicted its atomic mass to be 9.1 and valency, 2. Latter investigations proved him right.
Similarly, the atomic mass of uranium was corrected from 120 to 240. Corrections were also made in the atomic masses of gold, platinum, etc.
3. Predictions of properties of higher to undiscovered element : We know that Mendeleev classified the elements in order of their increasing atomic masses. However, this order had to be ignored at some places to make sure that the elements with similar properties fell in the same group. In doing so, he left some vacant places in the table. These vacant places were kept reserved for elements not discovered till then. Mendeleev was confident that these elements would be discovered later and they would occupy these vacant places. Not only this, he also predicted the properties of these undiscovered elements on the basis of this study of his the properties of the neighboring elements. Amazingly, when the missing elements of Mendeleev’s periodic table were discovered subsequently, their properties were found to be very similar to those predicted by Mendeleev.
The elements scandium, gallium and germanium were not known in 1871 but their existence was predicted by mendelev. He named these elements as eka-boron, eka-Aluminium and eka silicon when these elements were discovered, they were named scandium, gallium and germanium respectively and their properties were found to be in good agreement with those predicted by Mendeleev. Properties of ka-aluminium (predicted by Mendeleev) and those of the gallium (discovered later) are given in table.
Property | Eka-aluminium | Gallium |
Atomic mass | 68 | 69.7 |
Formula of oxide | E2O3 | Ga2O3 |
| Formula of chloride | ECl3 | GaCl3 |
Considering its atomic mass, titanium (Ti) should have been placed below aluminium in the periodic table, but Mendeleev placed is below silicon (Si) because the properties of titanium were similar to those of silicon. Thus, a gap was left below aluminium in the periodic table. This gap was filled up by gallium which was discovered later. The properties of gallium (Ga) were found to be similar to those of boron and aluminium.
4. Basic features intact : All the basic features of Mendeleev’s periodic table are intact even today. Even when a new class of elements, i.e., noble gases, were discovered, they found place in a separate group called the zero group. The existing order of the periodic table was not at all disturbed.
Discrepancies in Mendeleev’s periodic table :
Mendeleev’s periodic table has the following defects.
1. Position of hydrogen : The position of hydrogen in the periodic table is anomalous. Hydrogen resembles alkali metals (Li, Na, K, etc). So it may be placed in the group of the halogens (VII A)
2. Position of lanthanides and actinides : The elements from atomic number 57 to 71 are collectively known as lanthanides. They do not have a proper place in the periodic table. They all have been placed at the same position in group III and period 6. Similarly, the actinides (atomic numbers 89-103) also have no proper place in the periodic table. These elements have also been placed in the same position, in group III and period 7.
3. Some similar elements are separated, while some dissimilar elements have been placed in the group : Some similar elements are separated in the periodic table. For example, copper (Cu) and mercury (Hg), silver (Ag) and thallium (Tl), and barium (Ba) and lead (Pb). On the other hand, some dissimilar elements have been placed together in the same group. For example, copper (Cu), silver (Ag) and gold (Au) have been placed in group I along with the alkali metals. Similarly, manganese (Mn) is placed in the group of the halogens.
4. Presence of some anomalous pairs of elements : In Mendeleev’s periodic table, elements are arranged in order of increasing atomic mass. In some places, this order has been ignored.
(a) The atomic mass of argon is 40 and that of potassium is 39. But argon is placed before potassium in the periodic table.
(b) The positions of cobalt and nickel are not in proper order. Cobalt (at. mass = 58.9) is placed before nickel (at. mass = 58.6).
(c) Tellurium (at. mass = 127.6) is placed before iodine (at. mass = 126.9).
(d) Thorium (at. mass = 232.12) is placed before protactinium (at. mass = 231)
5. Position of isotopes : The isotopes of an element have no place in the periodic table.
The failure of Mendeleev’s periodic law to explain the wrong order of the atomic masses of some elements and the position of isotopes led scientists working in this field to conclude that atomic mass cannot be the basis for the classification of elements. There must be a more fundamental property of elements which can be the basis of classification.
Anomalous pairs of elements
Element → | Ar | K | Co | Ni | Te | I | Th | Pa |
Atomic mass | 40 | 39 | 59.9 | 58.6 | 127.6 | 126.9 | 232.12 | 231 |
| Group | 0 | IA | VIII | VIII | VI B | VII B | III B | III B |
