What is meant by a neutralization reaction?
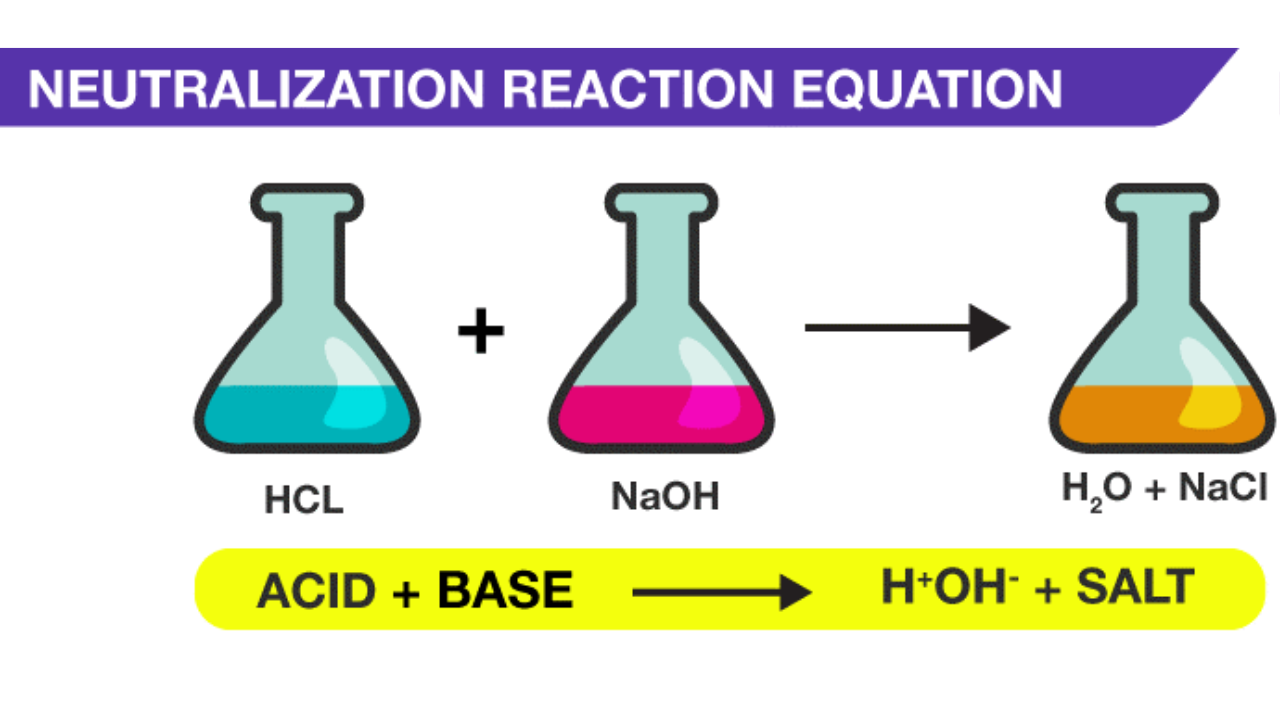
The reaction of an acid and a base is called a neutralisation reaction. In this reaction, the acidity of an acid is neutralised by an alkali. At the same time, the alkalinity of the alkali is neutralised by the acid. A salt and water are the only products of a neutralisation reaction.
Acid + alkali → salt + water
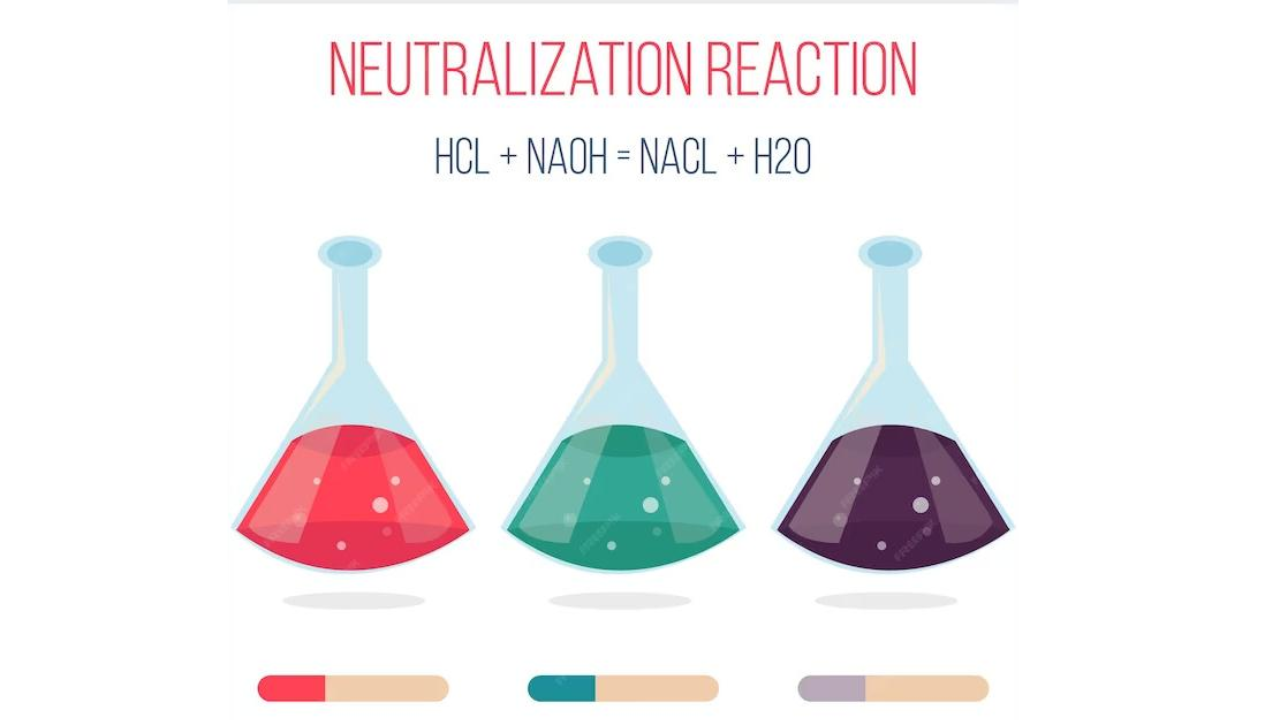
What happens in a neutralization reaction?
- A neutralisation reaction occurs when an alkali reacts with an acid to form a salt and water.
For example:

- (a) Acidic solutions contain hydrogen ions. Alkaline solutions contain hydroxide ions.
(b) When an alkali is added to an acid to neutralise the acid, it is the hydroxide ion which combines with the hydrogen ion and ‘cancel each other out’. A water molecule is formed.
H+(aq) + OH–(aq) → H2O(l)
The above ionic equation shows what happens in a neutralisation reaction. - When writing equations involving the neutralisation of ammonia solution by different acids, do not show the formation of water.
For example:
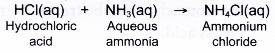
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution
What are the applications of neutralization in our everyday life
Neutralisation in agriculture
- Soils from different sources have different pH values. Soils can be acidic, neutral or alkaline.
- Farmers add powdered lime or limestone to neutralise acidity in soil.
- The traditional way of adding ashes of burnt wood is also effective. These ashes contain the alkalis such as sodium hydroxide and potassium hydroxide.
- Farmers use a compost of rotting vegetables or leaves to treat basic soils. As the vegetables and leaves decompose, carbon dioxide is given out. This acidic gas dissolves in water to form carbonic acid which can then neutralise the alkalis in basic soils.
Neutralisation in industry
- Electroplating industry
Effluent from the electroplating industry contains acids such as sulphuric acid and must be treated before it is discharged into rivers and streams. The acids in the effluent are neutralised by adding lime. - Energy industry
Power stations burn coal to produce electricity. Coals contain sulphur and burning sulphur in air produces sulphur dioxide which is responsible for the formation of acid rain.
S(s) + O2(g) → SO2(g)
When a mixture of coal and limestone is heated, the limestone decomposes to form lime.
CaCO3(s) → CaO(s) + CO2(g)
The lime neutralises the acidic sulphur dioxide.
2CaO(s) + 2SO2(g) + O2(g) → 2CaSO4(s) - Chemical industry
Sulphuric acid plants use the Contact process to produce sulphuric acid. Sulphur dioxide gas is produced from the combustion of sulphur or from the roasting of sulphide ores. To prevent sulphur dioxide gas from escaping into the atmosphere, waste gases are scrubbed by passing through powdered lime. Lime neutralises the acidic sulphur dioxide.
CaO(s) + SO2(g) → CaSO3(s)
Neutralisation in medicine
- Indigestion is caused by excess acid secreted by the stomach. It can be treated by neutralising the excess acid.
- Anti-acid indigestion medicines contain bases like calcium carbonate, sodium hydrogen carbonate, aluminium hydroxide and magnesium hydroxide to neutralise the hydrochloric acid in the stomach.
