What is meant by Latent Heat
Latent Heat
- Figure shows some ice cubes in a persons hand.
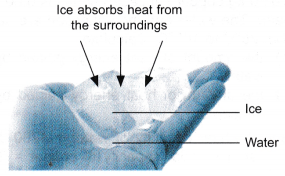 The person makes the following observations and inferences, as shown in Table.
The person makes the following observations and inferences, as shown in Table.Observation Inference The ice is colder than the surroundings. Heat energy is continuously being absorbed by the ice from the surroundings. The ice is at a constant temperature The heat energy absorbed by the ice does not cause an increase in temperature. The heat absorbed is not transferred to the molecules of ice as kinetic energy. The ice is melting There is change of phase from solid to liquid - Therefore, when ice melts, it absorbs heat without a change in its temperature.
- A similar situation is observed when heat is supplied continuously to boiling water. Placing a thermometer in the boiling water will show that its temperature remains constant.
- The heat energy which has to be supplied to change the state of a substance is called its latent heat. (or)
When a substance undergoes a change of phase such as melting or boiling, it absorbs heat without an increase in its temperature. The heat absorbed is known as latent heat. - From the principle of conservation of energy, we can infer that latent heat is released
(a) when a gas condenses at a constant temperature to become a liquid.
(b) when a liquid solidifies or freezes at a constant temperature to become a solid. - Table shows a summary of the processes and the latent heat involved.
Process Change of phase Occurs at Latent heat Melting Solid to liquid Melting point Absorbed Boiling Liquid to gas Boiling point Absorbed Condensation Gas to liquid Boiling point Released Solidification or freezing Liquid to solid Melting point or freezing point Released - The four main changes of phase are melting, boiling, condensation and solidification.
- Figure shows the four main changes and the latent heat involved.
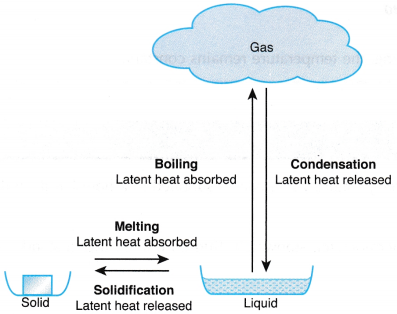
- The heating curve for a substance in the solid state when it is heated uniformly and undergoes a change of phase from solid to liquid to gas is as shown in Figure.
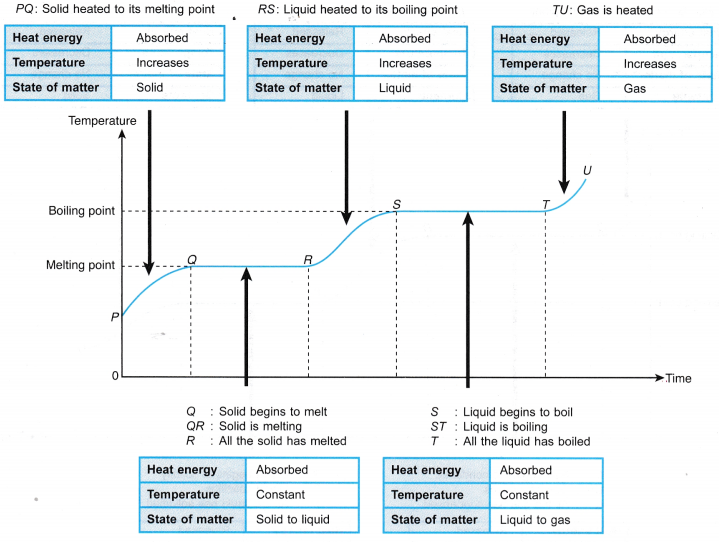
- The cooling curve for a substance in the gaseous state when it cools down and undergoes a change of phase from gas to liquid to solid is as shown in Figure.
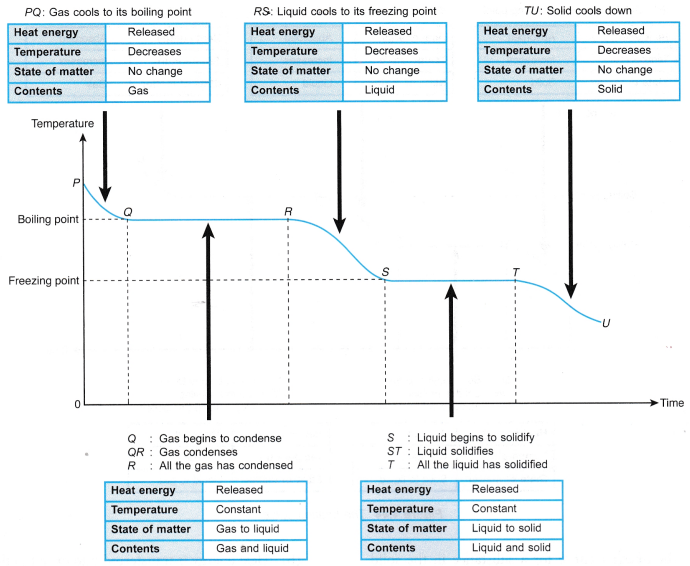
- There are three common characteristics when a substance undergoes a change of phase:
(a) The change of phase occurs at a particular temperature.
(b) Heat energy is transferred into or out of the substance during the change of phase.
(c) During the change of phase, the temperature remains constant even though there is transfer of heat. - The heat absorbed or the heat released at constant temperature during a change of phase is known as latent heat.
- The transfer of latent heat does not involve a change in the kinetic energy of the molecules.
Latent heat does not raise the temperature but latent heat has always to be supplied to change the state of a substance. The word ‘latent’ means ‘hidden’.
People also ask
- What is Heat Capacity?
- What is the Formula for Specific Heat Capacity?
- What is the latent heat of fusion of Ice?
- What is latent heat of vaporization of Water?
Latent Heat Experiment
1. Aim: To study the change in temperature when heat is supplied to a solid at its melting point.
Materials: Napthalene balls (mothballs) or octadecanol (stearyl alcohol), tap water
Apparatus: Boiling tube, 600 ml beaker, thermometer, stopwatch, Bunsen burner, tripod stand with wire gauze, retort stand with two clamps
Method:
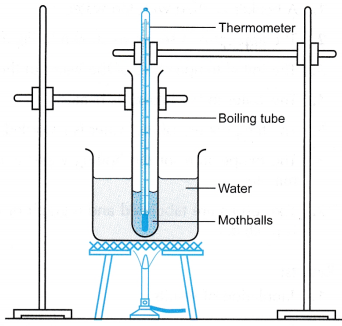
- Some mothballs are placed in the boiling tube until it is about one third full.
- The apparatus is set up as shown in Figure.
- The initial temperature of the mothballs is recorded.
- The mothballs are heated slowly in the water bath.
- The temperature of the mothballs is recorded every 30 s.
- The temperature is recorded for a few more minutes after the mothballs have started melting.
- The results are tabulated and a graph of temperature against time is plotted.
Results:
1. Tabulation of results.
| Time / s | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 | 330 | 360 | 390 | 420 |
| Temperature / °C | 25 | 43 | 58 | 70 | 80 | 84 | 87 | 89 | 89 | 89 | 89 | 89 | 89 | 92 | 98 |
2. Graph of temperature against time.
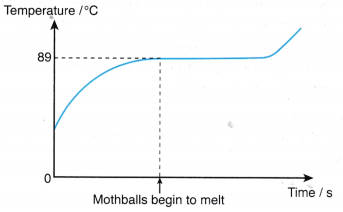
Discussion:
- Before melting, the temperature of the mothballs increases with time.
- At a temperature of about 89°C, the mothballs start to melt.
- During the melting process, the temperature remains constant although heat continues to be absorbed by the mothballs.
- After all the mothballs have melted, the temperature begins to increase again.
Conclusion:
When the mothballs are melting, the temperature remains constant.
2. Aim: To study the change in temperature when heat is supplied to a liquid at its boiling point.
Material: Tap water
Apparatus: 250 ml beaker, thermometer, stopwatch, Bunsen burner, tripod stand with wire gauze, retort stand with clamp
Method:
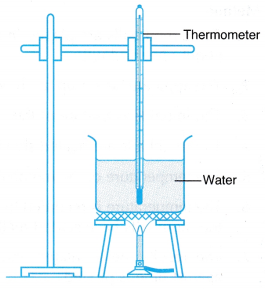
Caution: Make sure you stop the heating before the water dries up.
- A beaker is filled with tap water.
- The apparatus is set up as shown in Figure.
- The initial temperature of the water in the beaker is recorded.
- The water in the beaker is heated.
- The temperature of the water is recorded every 30 s.
- The temperature of the boiling water is recorded for a few more minutes.
- The results are tabulated and a graph of temperature against time is plotted.
Results:
1. Tabulation of results.
| Time / s | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 | 300 |
| Temperature / °C | 20 | 45 | 65 | 80 | 90 | 96 | 100 | 100 | 100 | 100 | 100 |
2. Graph of temperature against time.
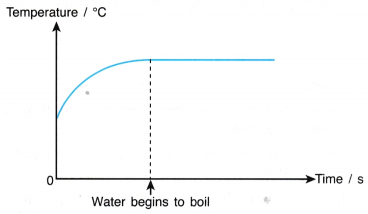
Discussion:
- Before boiling, the temperature of the water increases with time.
- At a temperature of about 100°C, the water starts to boil. Bubbles of steam are formed in the water.
- The temperature remains constant while the water is boiling although heat continues to be absorbed by the water.
Conclusion:
When the water is boiling, the temperature remains constant.
