Kerala SSLC Chemistry Previous Question Papers with Answers 2018 are part of Kerala SSLC Chemistry Previous Question Papers with Answers. Here we have given Kerala SSLC Chemistry Previous Question Papers with Answers 2018.
| Board | SCERT |
| Class | SSLC Class 10 |
| Subject | Chemistry |
| Category | Kerala SSLC Previous Question Papers |
Kerala SSLC Chemistry Previous Question Papers with Answers 2018 Free Download English Medium
Time Allowed: 1 1/2 Hours
Maximum Marks: 40
Instructions
- First 15 minutes is given as cool off time. This time is to be used for reading and understanding the questions.
- Answer the questions based on instructions.
- Answer the questions according to the score and time.
Attempt any four questions from 1 to 5. (Each question carries 1 score) (4 x 1=4)
Question 1:
The number of moles in 400 g CaCO3 is
[Hint: Gram atomic masses: Ca = 40 g, C = 12 g, O = 16 g]
Question 2:
Which of the following is a reversible reaction?
A: NaCl(aq) + AgNO3(aq) → NaNO3(aq)+AgCl(s)
B: NH4Cl(s) = NH3(g) + HCl(g)
Question 3:
Find the relation and fill in the blank.
Amino group: -NH2
Carboxylic group:
Question 4:
Which colour is given by cobalt oxides to glass?
Question 5:
The medicines which relieve pain are called ………..
Answer any four from questions 6 to 10. Each question carries 2 score. (4 x 2 = 8)
Question 6:
The balanced chemical equation for the formation of ammonia gas by the reaction between nitrogen gas and hydrogen gas is given.
N2 + 3H → 2NH3
a. Write the ratio between the number of moles of reactants and products in the correct order.
b. How many moles of ammonia are formed when 6 moles of N2 react with 6 moles of H2?
Question 7:
a. Which of the following statements is correct about chemical equilibrium?
i. At equilibrium both the reactants and products coexist.
ii. At equilibrium the rate of forward reaction is greater than the rate of backward reaction,
b. Write any one activity to increase the red colour in the following reaction.
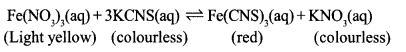
Question 8:
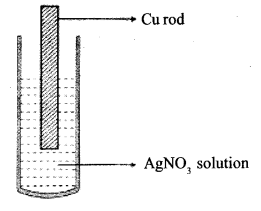
Observe the diagram showing a copper rod kept immersed in silver nitrate solution.
a. What is the colour change of the solution ?
b. Write the balanced chemical equation for the reaction.
Question 9:
a. Write an example for a metal which can be refined by liquation ?
b. What is calcination ?
Question 10:
Esters are obtained by the reaction between alcohols and carboxylic acids.
a. Write the chemical formula of ethyl ethanoate.
b. Write the chemical equation for the formation of ethyl ethanoate.
Answer any four from questions 11 to 15. Each question carries 3 score. (4 x 3 = 12)
Question 11:
a. What is gram atomic mass ?
b. Calculate the following:
i. How many gram atoms of sodium is present in 115 g sodium ?
ii. Mass of 5 gram atoms of calcium.
[Hint: Gram atomic masses: Na = 23 g, Ca = 40 g]
Question 12:
The outermost shell electronic configuration of an element ‘A’ (symbol given is not real) is 3s2 3p4.
a. To which period of the periodic table does this element belong to ?
b. Find the group number of the element.
c. Which is the block to which the element belongs ?
Question 13:
What happens to the rate of the forward reaction of the equilibrium,
2S02(g) + 02(g) ⇌ 2S03(g) + Heat during the following situations ?
a. increase in temperature
b. S03 is removed
c. pressure is decreased
Question 14:
a. What are isomers?
b. Write the structural formulae of any two position isomers of an alcohol with molecular formula C5H12O.
Question 15:
Petroleum is a mixture of different hydrocarbons.
a. Which method is used for separating the components of petroleum ?
b. Which is the hydrocarbon present in liquified petroleum gas (LPG) ?
c. Write any two environmental issues caused by the excessive consumption of fossil fuels.
Answer any four from questions 16 to 20. Each question carries 4 score. (4 x 4 = 16)
Question 16:
There are sub shells in shells around the nucleus.
a. What is the maximum number of electrons that can be accommodated in d-sub shell ?
b. Write the possible sub shells in 3rd shell in the increasing order of energy.
c. Which of the following is the outermost electronic configuration of copper ?
(Atomic number = 29)
A: 3d9 4s2
B: 3d10 4s1
Justify your answer.
Question 17:
Ions are the current carriers in electrolytes.
a. Sodium chloride in solid state is not an electrical conductor, but molten sodium chloride can conduct electricity. Give reason.
b. What are the products obtained at anode and cathode during the electrolysis of molten sodium chloride ?
c. If the aqueous solution of sodium chloride is subjected to electrolysis, what are the products obtained at each electrode?
Question 18:
Different methods are used for the concentration of ores.
a. What is the ore of aluminium ?
b. Explain how the ore of aluminium is concentrated by leaching.
Question 19:
Organic compounds are obtained through different chemical reactions.
a. What is the difference between substitution reaction and addition reactions ?
b. Complete the following reactions :
i. CH3 – CH3 = Cl2 → …………. + HCl
ii. CH3 – CH = CH – CH3HI → ……………..
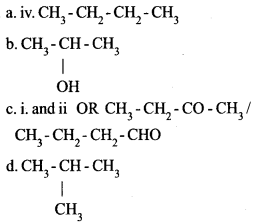
Question 20:
The structural formulae of some organic compounds are given below:
i. CH3 – CH2 – CO – CH3
ii. CH3 – CH2 – CH2 – CHO
iii. CH3 – CH2 – CH2 – OH
iv. CH3 – CH2 – CH2 – CH3
a. Which of these is an alkane ?
b. Write the structural formula of the position isomer of the third compound.
c. Which of the given compounds are functional isomers ?
d. Write the structural formula of the chain isomer of the fourth compound.
Answers
Answer 1:
4 or 400/100
Answer 2:
B : NH4Cl(s) = NH3(g) + HCl(g)
Answer 3:
-COOH
Answer 4:
Blue
Answer 5:
Anlagesics
Answer 6:
a. 1 : 3 : 2
b. 4
Answer 7:
a. At equilibrium, both the reactants and products coexist.
b. Add Fe(NO3)3 or KCNS into the system.
Answer 8:
a. The solution turns blue
b. Cu (s) + 2AgNO3(aq) → Cu(NO3)2 (aq) + 2Ag(s)
Answer 9:
a. Tin/Lead
b. Calcination is the process of heating the concentrated ore at a temperature below its melting point ton remove the volatile impurities.
Answer 10:
a. CH3 – COO – CH2 – CH3
b. CH3 – COOH + CH3 – CH2 – OH → CH3 – COO – CH2 – CH3+H2O
Answer 11:
a. Mass of an element equal to its atomic mass,
b. i. 5
ii. 5 x 40 g = 200 g
Answer 12:
a. 3
b. 16
c. p
Answer 13:
a. Rate of forward reaction decreases.
b. Rate of forward reaction increases.
c. Rate of forward reaction decreases.
Answer 14:
a. Compounds having same molecular formula but different chemical and physical proper¬ties are called Isomers.
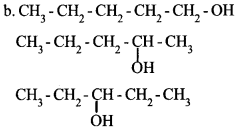
Answer 15:
a. Fractional distillation
b. Butane
c. CO – Poisoning
CO2 – Global warming
Answer 16:
a. 10
b. 3s < 3p < 3d
c. B: 3d10 4s1
To attain extra stability.
Answer 17:
a. In molten state or in aqueous solution, ions of the electrolytes can move freely. These ions are responsible for the conduction of electricity by the electrolytes. Sodium chloride in solid state is not an electrical conductor because its ions have no freedom of movement.
b. Sodium at Cathode, Chlorine from the Anode
c. Hydrogen at Cathode, Chlorine from the Anode
Answer 18:
a. Bauxite (Al2O3.2H2O)
b. Bauxite gets dissolved in hot concentrated NaOH. But the impurities does not dissolve in this. Only bauxite is soluble in hot oncentra- ted NaOH. As the impurities are insoluble, they can be easily filtered. This process is known is leaching.
Answer 19:
a. Reactions in which an atom or a group in a compound is replaced by another atom or a group are called substitution reactions.
Reactions in which unsaturated organic com¬pounds with double bond or triple bond re¬act with other molecules to form saturated compounds are called
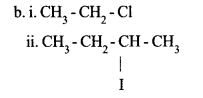
Answer 20:
We hope the Kerala SSLC Chemistry Previous Question Papers with Answers 2018 help you. If you have any query regarding Kerala SSLC Chemistry Previous Question Papers with Answers 2018, drop a comment below and we will get back to you at the earliest.
