Kerala SSLC Chemistry Previous Question Papers with Answers 2017 are part of Kerala SSLC Chemistry Previous Question Papers with Answers. Here we have given Kerala SSLC Chemistry Previous Question Papers with Answers 2017.
| Board | SCERT |
| Class | SSLC Class 10 |
| Subject | Chemistry |
| Category | Kerala SSLC Previous Question Papers |
Kerala SSLC Chemistry Previous Question Papers with Answers 2017 Free Download English Medium
Time Allowed: 1 1/2 Hours
Maximum Marks: 40
Instructions
- Fifteen minutes are allowed as cool off time. This time is to be used for reading the questions and planning the order for writing answers.
- Write answers only after clearly reading the questions and instructions.
- While writing answers, consider the score and the time required.
Question 1:
This question has choice. Answers any one of them only.
A. The electrons in atoms are arranged in the sub shells.
a. Which are the sub shells present in the third shell or the M shell? 1
b. Complete the table given below, (symbols given are not real) 3
| Element | Sub Shell electronic configuration | Highest shell number in the sub shell electronic configuration | Period |
| 5X | Is² 2s² 2p¹ | 2 | 2 |
| 11Y | Is² 2s² 2p6 3s¹ | 3 | …. ii ….. |
| 19Z | ………. i ………. | 4 | …. iii ….. |
OR
B. The sub shell electronic configuration of an element is given as [Ar]3d5 4s1.
a. How many shells of this element has electrons in it? 1
b. Which is the sub shell to which the last electron is added? 1
c. What is the atomic number of the element? 1
d. What is the group number of the element? 1
Question 2:
This question has choice. Answer any one of them only.
A. Atomic mass of H = 1 and O = 16
a. L Calculate the gram molecular mass of O2. 1
ii. How many molecules are present in 16 gram of O2? 1
b. In the reaction
2H2 + O2 → 2H2O
i. How many moles of 02 are required to produce 10 moles of H2O ? 2
ii. What volume of 02 gas at STP is required to produce 2 moles of water ? 1
OR
B. a. Calculate the number of molecules in each of the following:
i. 22.4 L of CO2 gas at STP 1
ii. 4 g of H2 (atomic mass of H= 1) 2
b. The gram molecular mass of glucose (C6H12O6) is 180 g. Find the mass of glucose dissolved in 500 ml of 1 M solution of glucose. 2
Question 3:
See the figure given below.
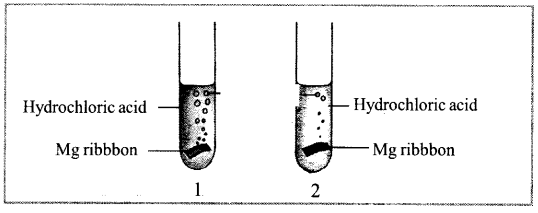
The Mg (Magnesium) ribbons are of equal mass and size. The reaction is faster in test tube 1 and slower in test tube 2. Now answer the following questions.
a. Give reason for the faster rate of reaction in test tube 1 than in the test tube 2. 1
b. Write balanced chemical equation for the reaction. 1
Question 4:
Examine the reaction
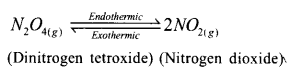
a. Which of these is a brown coloured gas? 1
b. How does the colour of the brown gas change when a test tube filled with this gas and closed with a cork is immersed in
i. A vessel containing ice pieces. 1
ii. A vessel containing hot water. 1
Question 5:
A freshly cut piece of sodium when exposed to air for sometime looses its lustre in the freshly cut portion.
a. What may be the reason for this? 1
b. Write any two chemical equations to substantiate your answer. 2
Question 6:
The reactivity series of a few metals are given below:
Mg > Zn > Fe > Cu > Ag
a. What happens
i. When a piece of magnesium (Mg) is dipped in copper sulphate (CuSO4) solution. 1
ii. When a piece of silver (Ag) is dipped in Zinc sulphate (ZnSO4) solution. 1
b. If a galvanic cell is constructed using Fe and Ag electrodes, which will be the positive electrode? 1
Question 7:
Bauxite (Al2O3,2H2O) and clay (Al2O3. SiO2 2H2O) are two naturally occurring minerals of aluminium,
a. Which one of these is an ore of aluminium? 1
b. Give two reasons for your answer. 2
Question 8:
Electricity and Carbon monoxide (CO) are reducing agents used to extract metals from their ores.
a. Which of these is used to extract sodium from sodium chloride? Why? 2
b. Which reducing agent is used to extract iron from haematitie (Fe2O3)? 1
Question 9:
a. Write IUPAC name of the following hydrocarbon: 1
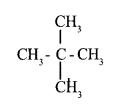
b. Write the structural formula of the compound given below 1
3 -Ethyl-2-Methylhexane
Question 10:
Examine the structural formulae of the compounds given below and tabulate all the isomerpairs.
Name the type of isomerism in each pair. 4
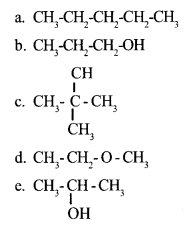
Question 11:
a. Complete the following equations by writing the formula/name of the products. 3
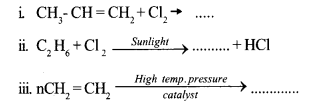
b. Write balanced equation for the combustion of the fuel propane (C3H8). 2
Question 12:
Chemistry contributes a lot in allopathic medicines. What are the functions of the following types of medicines? 3
a. Analgesics
b. Antibiotics
c. Antipyretics
Answers
Answer 1:
1. A
a. s, p, d
b. i. 1s² 2s² 2p63s2 3p64s1
ii. 3
iii. 4
1. B
a. 4 b. d
c. 24 d. 6
Answer 2:
A. a. i. 32 g
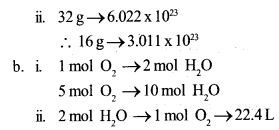
B. a.i. 22.4L CO2 gas at STP =1 mol = 6.022 x 1023
ii. No.of molecules in 2 g Hydrogen molecules = 6.022 x 1023 No of molecules in 4g Hydrogen =2 x 6.022 x 1023
b. No.of moles of glucose in 500 ml 1M glucose = 0.5
Mass of 0.5 mol glucose = 90 g
Answer 3:
a. Concentrated HC1 was used in Test tube
1 hence the rate of reaction is faster
b. Mg (s) + 2 HCl (aq) → MgCl2 (aq) + H2(g)
Answer 4:
a. NO2
b. i. Brown colour fades
ii. Brown colour increases.
Answer 5:
a. Oxygen, watervapour and CO2 in the atmosphere reacts with sodium and form compounds.
b. 4 Na(s) + O2 (g) → 2Na2O
2Na(s) + 2H2O(g) → 2NaOH(g)+H2(g)
Answer 6:
a. i. The metal copper gets displaced.
ii. Substitution does not take place
b. Ag
Answer 7:
a. Bauxite
b. A mineral from which a metal is economically, easily and quickly extracted, is called the ore of the metal. Among all minerals of aluminium Bauxite possesses these properties. Hence bauxite is the ore of Aluminium.
Answer 8:
a. Electricity . Metals which have high reactivity are separated from their ore by electricity which is a strong reducing agent,
b. CO
Answer 9:
a. 2,2 – dimethyl propane
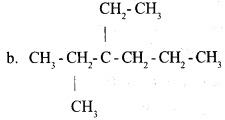
Answer 10:
b, d – Functional Isomerism
b, e – Position Isomerism
a, c – Chain Isomerism
Answer 11:
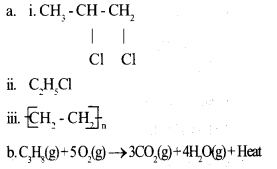
Answer 12:
a. To relieve pain
b. To destroy the disease causing micro organisms and prevent their growth.
c. To lower body temperature.
We hope the Kerala SSLC Chemistry Previous Question Papers with Answers 2017 help you. If you have any query regarding Kerala SSLC Chemistry Previous Question Papers with Answers 2017, drop a comment below and we will get back to you at the earliest.
