Kerala SSLC Chemistry Model Question Papers with Answers Paper 2 are part of Kerala SSLC Chemistry Previous Year Question Papers with Answers. Here we have given Kerala SSLC Chemistry Model Question Papers with Answers Paper 2.
| Board | SCERT |
| Class | SSLC Class 10 |
| Subject | Chemistry |
| Category | Kerala SSLC Previous Question Papers |
Kerala SSLC Chemistry Model Question Papers with Answers Paper 2 Free Download English Medium
Time Allowed: 1 1/2 Hours
Maximum Marks: 40
Instructions
- First 15 minutes is cool-off time.
- Read all questions carefully.
- Questions with scores 1, 2, 3 and 4 are categorised separately.
- 5 questions are given in each category. Answer any 4 questions from each category.
- Answer each question by keeping time.
Answer any 4 questions from 1 to 5 (1 score each)
Question 1:
Glass is an important man-made material. Which tyope of glass is used to make lenses and prisms?
Question 2:
Some subshells are given. Select the incorrect subshells.
(a) 2p
(b) 3f
(c) 4d
(d) 2d
Question 3:
Fill the relation and fill in the blank
Bauxite : Aluminium
Magnetite : _____
Question 4:
5 – 8% solution of ethanol is known as _____
Question 5:
Write the IUPAC name of the following compound.
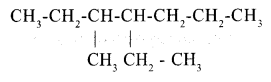
Answer any 4 questions from 6 to 10 (2 scores each)
Question 6:
The metallurgy of aluminium involves two stages – a concentration process and electrolysis.
a. Why is the ore of aluminium treated with hot concentrated NaOH?
b. During electrolysis, cryolite is added to the electrolyte. Why?
Question 7:
Given below are the IUPAC names of two organic compounds.
a. 3, 3, 5, 5 – tetra methyl octane
b. 4-methyl hept-2-yne Draw their structures.
Question 8:
PVC is an extensively used polymer.
a. Which is the monomer of PVC?
b. Give the structure of PVC.
Question 9:
Fill in the blanks in the table given below.
| Cell | Equation | Reaction | Equation | Reaction |
| Mg-Cu | Mg→Mg2++2e– | …………… | ………….. | Reduction |
| Cu-Ag | ………….. | Oxidation | Cu2++2e– →Cu | …………. |
Question 10:
The symbols of some elements are given below.
(Th, Mg, K, F, Mn)
(a) The fuel used in nuclear reactor
(b) The element which shows + 1 oxidation state
Answer any 4 questions from 11 to 15 (3 scores each)
Question 11:
250 mL of potassium chloride (KCl) solution contains 3.725 g of KCl.
a) Calculate the concentration of the solution.
b) Calculate the number of atoms present in 3.725 g of KCl. (K – 39, Cl – 35.5)
Question 12:
FeCl2, FeCl3 are the two compounds of iron.
a) Calculate the oxidation states of iron in these compounds.
(The oxidation state of Cl is l–)
b) Explain the reason for exhibiting such variable valancies.
(The atomic number of Fe – 26)
Question 13:
When electricity is passed through acidified water that undergo decomposition. Complete the equations related to this process
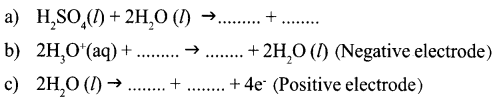
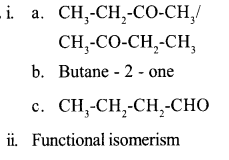
Question 14:
i The functional groups of two compounds with same molecular formula are given.
Analyse it and complete the boxes.
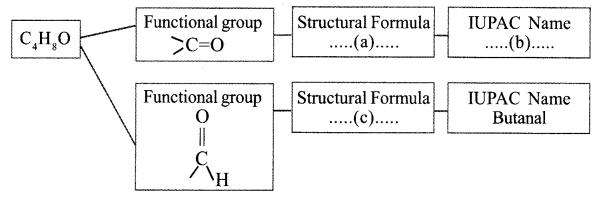
ii. What is the name of this isomerism?
Question 15:
The outermost electronic configuration of element ‘X’ is 3s23p1. Answer the questions given below related to this element, (symbol of the elelment is not actual).
a. What is the atomic number of this element?
b. Write the group number and period number of X?
c. Write the formula of the oxide of this element?
Question 16:
The chemical change taking place in an electroyte when electricity is passed through it is electrolysis.
a. Which are the products of electrolysis of aqueous CuCl2 using graphite electrodes?
b. Write the electrode reactions involved in the electrolysis of aqueous CuCl2 using graphite electrodes?
c. What is the charge of the anode in the electrolytic cell?
Question 17:
The pictorial representation of some experiments are given below.
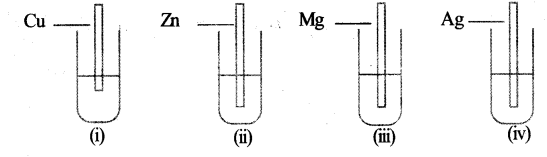
All the test tubes contain FeSO4 solution.
(a) In which of these test tubes, reaction will take place?
(b) What is the name given to such reactions?
(c) Write the equations of the reactions.
Question 18:
One of the stages of the manufacture of sulphuric acid in contact process is given below.
2SO2 (g) + O2(g) ⇌ 2SO3 (g) + heat
(a) Which catalyst is applied in this process?
(b) What is the function of such catalysts in a reversible reactions?
(c) Write the name and molecular formula of the compound formed when SO3 gas is dissolved in sulphuric acid H2 SO4 .
(d) How sulphuric acid is prepared from this solution? Write the equation
Question 19:
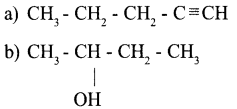
Write the structures of the following compounds,
(a) Hex – 1 – yne
(b) Butane – 2 – o1
(c) 3 – Ethyl – 2,2 – dimethylpentane
(d) Butanone
Question 20:
The picture of an electrochemical cell is given
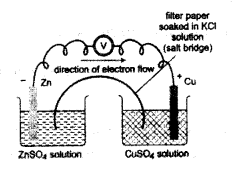
a. Identify the anode and cathode of this cell.
b. Write the chemical reactions taking place in both the electrodes
c. Write the redox reaction taking place in this cell
Answers
Answer 1:
Flint glass
Answer 2:
3f
Answer 3:
Iron
Answer 4:
Wash
Answer 5:
4-ethyl-3-methyl heptane
Answer 6:
a. To convert Aluminium oxide to Sodium aluminate.
b. To dissolve Alumina
Answer 7:
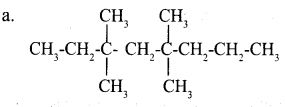
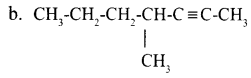
Answer 8:
a. Vinyl chloride
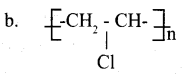
Answer 9:
| Cell | Equation | Reaction | Equation | Reaction |
| Mg-Cu | Mg→Mg2++2e– | Oxidation | Cu2++2e–→Cu | Reduction |
| Cu-Ag | Ag+ + 1e–→Ag | Oxidation | Cu2++2e–→Cu | Reduction |
Answer 10:
(a) Th
(b) K
Answer 11:

![]()
(b) The number of molecules in 0.05
moles of KCl = 0.05 x 6022 x 1023
∴ Number of atoms
= 0.05 x 6.022 x 1023 x 2
= 0.1 x 6.022 x 1023
Answer 12:
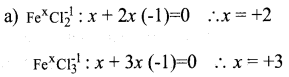
(b) The electronic configuration of Fe:
1s22s22p63s23p63d64s2
In this the energy of 4s electrons and 3d electrons are almost equal. During the formation of FeCl2 the electrons from 4s only take partion the reaction. But during the formation of FeCl3 both the 4s and 3d electrons do take part in the reaction.
Therefore, Fe shows two different oxidation states.
Answer 13:
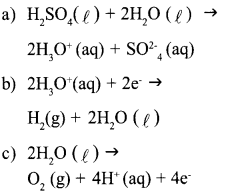
Answer 14:
Answer 15:
a. Complete electronic configuration
1s2, 2s2, 2p6, 3s2, 3p1
Atomic number = 13
b. Group number of X – 13
Period – 3
c. Valency of X -3
Valency of Oxygen – 2
Oxide of the Element – X2O2
Answer 16:
a. Chlorine, Copper
b. Anode
Cl– + e– → Cl
Cl + Cl → Cl2
Cathode : Cu2+ + 2e– → Cu
c. Positive
Answer 17:
a) (ii), (iii) in these test tubes reaction will take place.
b) These reactions are called substitution reactions.
c) Zn(s) + FeSO4(aq) →
ZnSO4 (aq) + Fe(s)
Mg(s) + FeSO4(aq) →
MgSO4 (aq) + Fe(s)
Answer 18:
(a) Vanadium pentoxide (V2O5)
(b) When a catalyst is applied in a re-versible reaction, the speed of both the forward and backward reactions are increased. Thus the equilibrium is attained more earlier.
(c) Oleum (H2S2O7)
d) H2S2O7 + H2O → 2H2SO4
(Sulphuric acid is prepared by dilut¬ing oleum with water)
Answer 19:
We hope the Kerala SSLC Chemistry Model Question Papers with Answers Paper 2 help you. If you have any query regarding Kerala SSLC Chemistry Model Question Papers with Answers Paper 2, drop a comment below and we will get back to you at the earliest.
