ISC Chemistry Previous Year Question Paper 2019 Solved for Class 12
Maximum Marks: 70
Time allowed: 3 hours
- Candidates are allowed additional 15 minutes for only reading the paper.
- They must NOT start writing during this time.
- All questions are compulsory.
- Question 1 is of 20 marks having four subparts, all of which are compulsory.
- Question numbers 2 to 8 carry 2 marks each, with two questions having internal choice.
- Question numbers 9 to 15 carry 3 marks each, with two questions having an internal choice.
- Question numbers 16 to 18 carry 5 marks each, with an internal choice.
- All working, including rough work, should be done on the same sheet as, and adjacent to the rest of the answer.
- The intended marks for questions or parts of questions are given in brackets [ ].
- Balanced equations must be given wherever possible and diagrams where they are helpful.
- When solving numerical problems, all essential working must be shown.
- In working out problems, use the following data:
- Gas constant R = 1.987 cal deg-1 mol-1 = 8.314 JK-1 mol-1 = 0.0821 dm3 atm K-1 mol-1
- 11 atm = 1 dm3 atm = 101.3 J. 1 Faraday = 96500 coulombs.
- Avogadro’s number = 6.023 × 1023.
Question 1.
(a) Fill in the blanks by choosing the appropriate word/words from those given in the brackets: [4 × 1]
(more than, primary, cathode, Lucas reagent, two, four, less than, Grignard’s reagent, tertiary, anode, zero, equal to, three)
(i) The elevation of a boiling point of 0.5 M K2SO4 solution is ……….. that of 0.5 M urea solution. The elevation of the boiling point of 0.5 M KCl solution is …………. that of 0.5 M K2SO4 solution.
(ii) A mixture of conc. HCl and anhydrous ZnCl2 is called ……….. which shows maximum reactivity with ……….. alcohol.
(iii) In electrolytic refining, the impure metal is made ………. while a thin sheet of pure metal is used as ………..
(iv) When the concentration of a reactant of the first-order reaction is doubled, the rate of reaction becomes ………… times, but for a ……… order reaction, the rate of reaction remains the same.
(b) Select the correct alternative from the choices given: [4 × 1]
(i) The cell reaction is spontaneous or feasible when emf of the cell is:
(1) negative
(2) positive
(3) zero
(4) either positive or negative
(ii) Which, among the following polymers, is a polyester:
(1) melamine
(2) bakelite
(3) terylene
(4) polythene
(iii) The correct order of increasing acidic strength of the oxoacids of chlorine is:
(1) HCIO3 < HClO4 < HClO2 < HClO
(2) HClO < HClO2 < HClO3 < HClO4
(3) HClO2 < HClO < HClO4 < HClO3
(4) HClO3 < HClO4 < HClO < HClO2
(iv) A catalyst is a substance which:
(1) changes the equilibrium constant of the reaction
(2) increases the equilibrium constant of the reaction
(3) supplies energy to the reaction
(4) shortens the time to reach equilibrium.
(c) Match the following: [4 × 1]
| (i) Diazotisation | (a) Anisotropic |
| (ii) Crystalline solid | (b) Reimer-Tiemann reaction |
| (iii) Phenol | (c) Diphenyl |
| (iv) Fitting reaction | (d) Aniline |
(d) Answer the following questions: [4 × 2]
(i) (1) Which trivalent ion has a maximum size in the Lanthanoid series i.e., Lanthanum ion (La3+) to Lutetium ion (Lu3+)?
(at. no. of Lanthanum = 57 and Lutetium = 71)
(2) Explain why Cu2+ is paramagnetic but Cu+ is diamagnetic.
(at. no. of Cu = 29)
(ii) When a coordination compound C0Cl3.6NH3 is mixed with AgNO3, three moles of AgCl are precipitated per mole of the compound. Write the structural formula and IUPAC name of the coordination compound.
(iii) Calculate the boiling point of urea solution when 6 g of urea is dissolved in 200 g of water. (Kb for water = 0.52 K kg mol-1, boiling point of pine water = 3 73 K, mol. wt. of urea = 60)
(iv) Identify the compounds A, B, C and D in the given reaction:
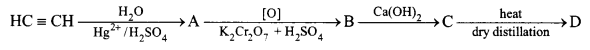
Answer:
(a) (i) more than, less than
(ii) Lucas reagent, tertiary
(iii) anode, cathode
(iv) two, zero
(b) (i) → (2)
(ii) → (3)
(iii) → (2)
(iv) → (4)
(c) (i) (d)
(ii) (a)
(iii) (b)
(iv) (c)

Cu2+ has one unpaired electron. Therefore, it is paramagnetic. Cu+ has no unpaired electrons. Therefore, it is diamagnetic.
(ii) [CO(NH3)6]Cl3
hexamine cobalt (III) chloride
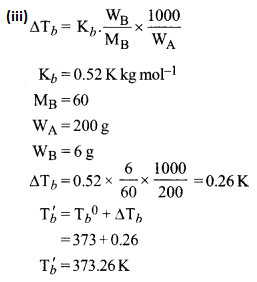
(iv) A – CH3CHO, Acetaldehyde
B – CH3COOH, Acetic acid
C – (CH3COO)2Ca, Calcium acetate
D – CH3COCH3, Acetone
Question 2. [2]
(a) For the reaction A + B → C + D, the initial rate for different reactions and initial concentration of reactants are given below:
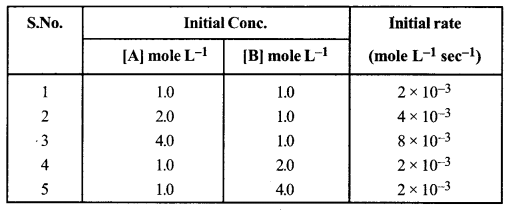
(i) What is the overall order of reaction?
(ii) Write the rate law equation.
or
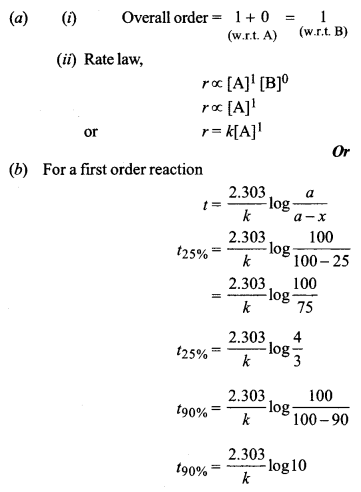
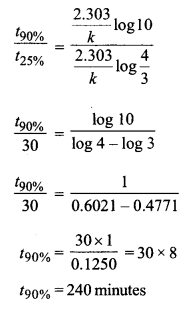
(b) 25% of a first-order reaction is completed in 30 minutes. Calculate the time taken in minutes for the reaction to go to 90% completion.
Answer:
Question 3. [2]
- Name the type of drug which lowers the body temperature in high fever condition.
- What are tranquilizers? Give one example of tranquilizer.
Answer:
- Antipyretics
- Tranquilizers: These are the drugs which are used for the treatment of stress, fatigue, mild and severe mental diseases.
For example phenelzine
Question 4. [2]
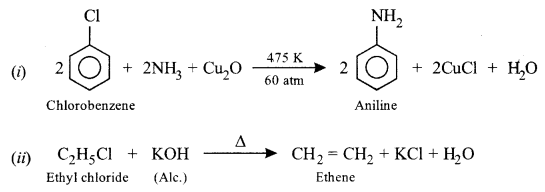
Write the balanced chemical equation for each of the following:
(i) Chlorobenzene treated with ammonia in the presence of Cu2O at 475 K and 60 atm.
(ii) Ethyl chloride treated with alcoholic potassium hydroxide.
Answer:
Question 5. [2]
- Name the monomer and the type of polymerisation that takes place when PTFE is formed.
- Name the monomers of nylon 6, 6.
Answer:
- Tetrafluoroethylene, F2C = CF2
Addition polymerisation - Monomers of nylon – 6, 6 are hexamethylene diamine and adipic acid.
Question 6. [2]
Name two water-soluble vitamins and the diseases caused by their deficiency in the diet of an individual.
Answer:
Two water-soluble vitamins are
- Vitamin C, Deficiency disease – Haemorrhage
- Vitamin B1 – Deficiency disease – Beriberi
Question 7. [2]
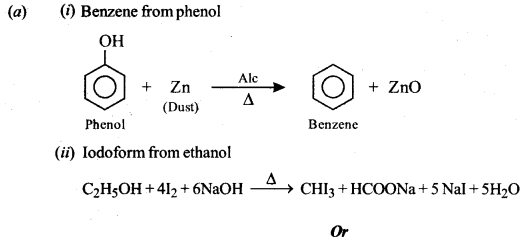
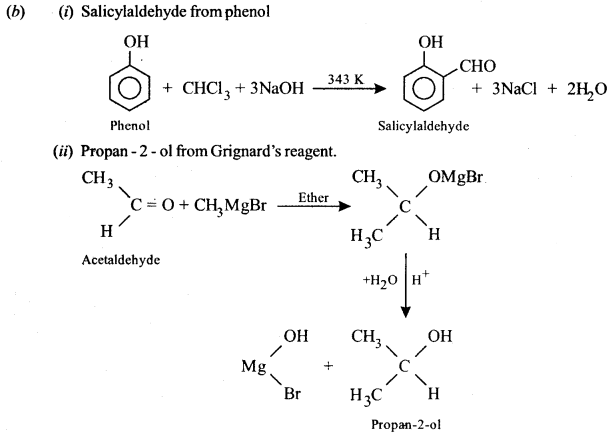
(a) How will you obtain the following? (Give balanced chemical equations):
(i) Benzene from phenol.
(ii) Iodoform from ethanol.
Or
(b) How will you obtain the following? (Give balanced chemical equations):
(i) Salicylaldehyde from phenol.
(ii) Propan-2-ol from Grignard’s reagent.
Answer:
Question 8.
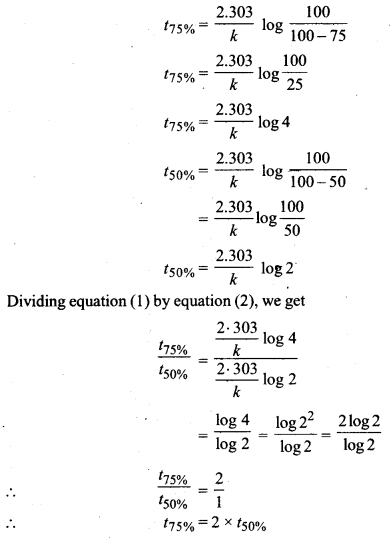
Show that for a first-order reaction the time required to complete 75% of reaction is about 2 times more than that required to complete 50% of the reaction.
Answer:
For a first-order reaction
\(t=\frac{2 \cdot 303}{k} \log \frac{a}{a-x}\)
Question 9. [3]
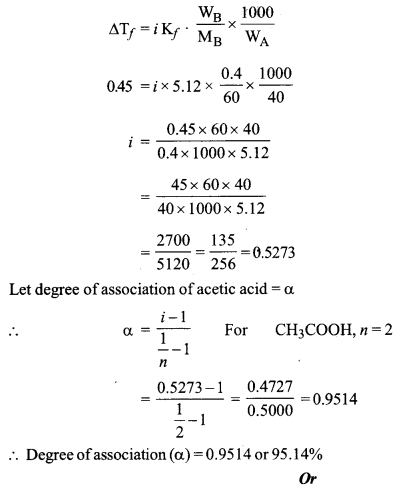
(a) When 0.4g of oxalic acid is dissolved in 40g of benzene, the freezing point of the solution is lowered by 0.45 K. Calculate the degree of association of acetic acid. Acetic acid forms dimer when dissolved in benzene.
(Kf for benzene = 5.12 K kg mol-1, at. wt. C = 12, H = 1, O = 16)
Or
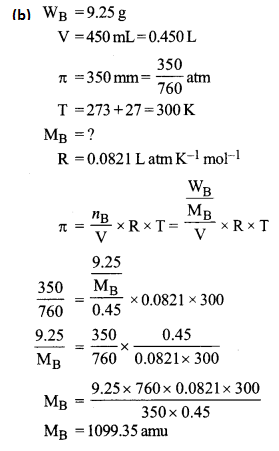
(b) A solution is prepared by dissolving 9.25 g of non-volatile solute in 450 mL of water. It has an osmotic pressure of 350 mm of Hg at 27°C. Assuming the solute is non-electrolyte, determine its molecular mass. (R = 0.0821 lit atm K-1 mol-1)
Answer:
(a) ∆Tf = 0.45 K
i = ?
Kf = 5.12 K kg mol-1
MB of CH3COOH = 60
WA = 40 g
WB = 0.4 g
We know that
Question 10. [3]
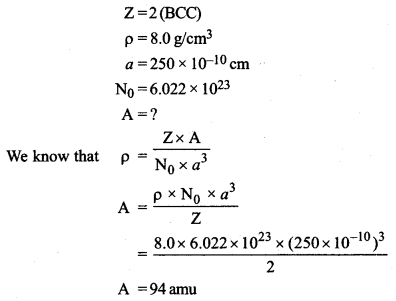
An element occurs in a body-centred cubic structure. Its density is 8.0 g/cm3. If the cell edge is 250 pm, calculate the atomic mass of an atom of this element. (N0 = 6.023 × 1023)
Answer:
Question 11. [3]
Describe the role of the following:
(i) Cryolite in the extraction of aluminium from pure alumina.
(ii) NaCN in the extraction of silver from silver ore.
(iii) Coke in the extraction of iron from its oxides.
Answer:
(i) (a) It lowers the melting point of bauxite.
(b) It increases the conductivity of the electrolyte.
(ii) It reacts with the silver ore, Ag2S, (Argentite) to form soluble complex from which silver can be displaced by adding more electropositive metal like Zn
\(\begin{array}{l}{\mathrm{Ag}_{2} \mathrm{S}+4 \mathrm{NaCN}+2 \mathrm{O}_{2} \rightleftharpoons 2 \mathrm{Na}\left[\mathrm{Ag}(\mathrm{CN})_{2}\right]+\mathrm{Na}_{2} \mathrm{SO}_{4}} \\ {2 \mathrm{Na}\left[\mathrm{Ag}(\mathrm{CN})_{2}\right]+\mathrm{Zn} \longrightarrow \mathrm{Na}_{2}\left[2 \mathrm{n}(\mathrm{CN})_{4}\right]+2 \mathrm{Ag} \downarrow}\end{array}\)
(iii) In the extraction of iron from its oxide, coke acts as
(a) fuel
(b) Reducing agent
Question 12. [3]
(i) Write the IUPAC names of the following:
(1) K3[Fe(C2O4)3]
(2) [Co(NH3)5Cl]SO4
(ii) [Fe(CN)6]4- is a coordination complex ion.
(1) Calculate the oxidation number of iron in the complex.
(2) Is the complex ion diamagnetic or paramagnetic?
(3) What is the hybridisation state of the central metal atom ?
(4) Write the IUPAC name of the complex ion.
Answer:
(i) (1) potassium trioxalatoferrate (III)
(2) pentaamminechloridocobalt (III) sulphate
(ii) (1) x + 6(-1) = -4 ⇒ x = +2
(2) Fe2+ (3d6) has no unpaired electrons, it is diamagnetic
(3) d2sp3
(4) hexacyanoferrate (II) ion.
Question 13. [3]
(a) Explain why:
(i) Transition elements form alloys.
(ii) Zn2+ salts are white whereas Cu2+ salts are coloured.
(iii) Transition metals and their compounds act as a catalyst.
Or
(b) Complete and balance the following chemical equations:
(i) KMnO4 + H2SO4 + H2C2O4 → ____ + ____ + ____ + _____
(ii) K2Cr2O7 + H2SO4 + KI → ____ + _____ + _____ + _____
(iii) K2Cr2O7 + H2SO4 + FeSO4 → _____ + _____ + _____ + _____
Answer:
(a) (i) This is because transition metals have similar atomic radii and form substitutional alloys.
(ii) Zn2+(3d10) salts have no impaired electrons but Cu2-(3d9) salts have one unpaired electrons. Hence Zn2+ salts are white while Cu2- salts are coloured.
(iii) This is because of transition metals
- have a number of unpaired electrons in their (n-1) d-orbitals
- can show variable oxidation states.
- form intermediates and provide a new reaction path having lower energy of activation.
- they can adsorb one of the reactants on their surfaces.
Or
(b) (i) 2KMnO4 + 3H2SO4 + 5H2C2O4 → K2SO4 + 2MnSO4 + 8H2O + 10CO2
(ii) K2Cr2O7 + 7H2SO4 + 6KI → 4K2SO4 + Cr2(SO4)3 + 3I2 + 7H2O
(iii) K2Cr2O7 + 7H2SO4 + 6FeSO4 → 4K2SO4 + Cr2(SO4)3 + 3I2 + 7H2O
Question 14. [3]
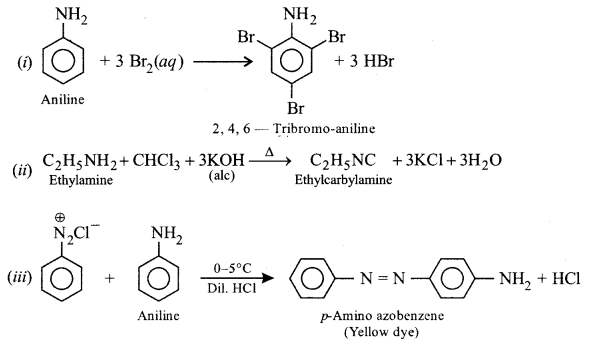
Give balanced equations for the following:
(i) Aniline is treated with bromine water.
(ii) Ethylamine is heated with chloroform and alcoholic solution of potassium hydroxide.
(iii) Benzene diazonium chloride is treated with ice cold solution of aniline in acidic medium.
Answer:
Question 15.
Define the following terms with suitable examples:
(i) Peptisation
(ii) Electrophoresis.
(iii) Dialysis
Answer:
(i) Peptisation: It is the process of conversion of fresh precipitate into colloidal particles by shaking it with dispersion medium in the presence of a small amount of suitable electrolyte.
e.g., a reddish-brown coloured colloidal solution is obtained from fresh precipitate of Fe(OH)3 by shaking it with a small quantity of a dilute solution of FeCl3.
(ii) Electrophoresis: It is the movement of colloidal particles under the influence of the electric field, e.g., when a positive charged ferric hydroxide sol is subjected to an electric field, the colloidal particles migrate towards cathode.
(iii) Dialysis: It is the process of separation of the colloidal particles from those of crystalloids by diffusion of the mixture through a semipermeable membrane e.g., blood is purified in the kidneys because walls of the kidneys are semipermeable.
Question 16. [5]
(a) (i) Calculate the mass of silver deposited at cathode when a current of 2 amperes is passed through a solution of AgNO3 for 15 minutes.
(at. wt. of Ag= 108, 1F = 96500 C)
(ii) Calculate the emf and ΔG for the cell reaction at 298 K
Mg(s)|Mg2+(0.1 M) || CU2+(0.01 M)|Cu(s)
Given E°cell = 2.71 V
1F = 96,500 C
Or
(b) (i) Define the following terms:
(1) Specific conductance
(2) Kohlrausch’s Law
(ii) The resistance of a conductivity cell containing 0.001 M KCl solution at 298 K is 1500 ohm. What is the cell constant and molar conductivity of 0.001 M KCl solution, if the conductivity of this solution is 0.146 × 10-3 ohm-1cm-1 at 298 K?
Answer:
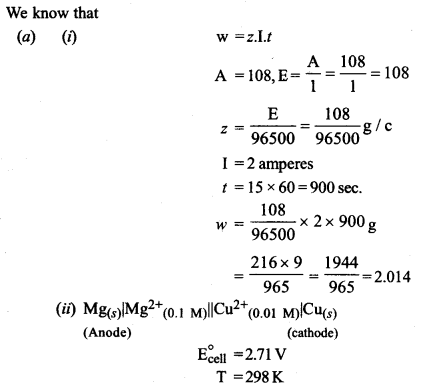
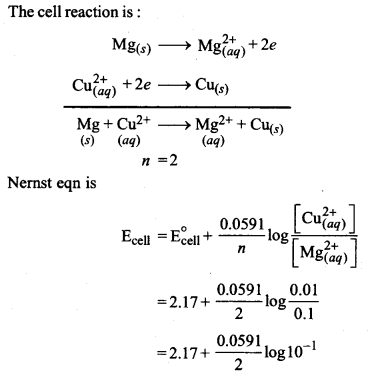
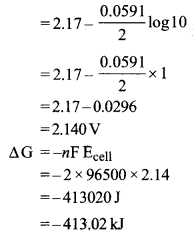
Or
(b) (i) (1) It is the conductivity power of all the ions present in ICC of the solution of the electrolyte.
(2) It states that at infinite dilution, the molar conductance of an electrolyte is the sum of molar conductances of its ions with molar conductance of each ion multiplied with the number of ions present in the formula of the electrolyte.
(ii) R = 1500 ohms
C = 0.001 M
k = 0.146 × 10-3 ohm-1cm-1
k = conductance × cell constant
\(\begin{aligned} 0.146 \times 10^{-3} &=\frac{1}{\mathrm{R}} \times \text { cell constant } \\ &=\frac{1}{1500} \times \text { cell constant } \end{aligned}\)
cell constant = 0.146 × 10-3 × 1500
= 0.146 × 15 × 10-1
= 0.146 × 1.5
cell constant = 0.2190 ohm-1cm-1
Question 17. [5]
(a) (i) Explain why:
(1) Fluorine has lower electron affinity than chlorine.
(2) Red phosphorus is less reactive than white phosphorus.
(3) Ozone acts as a powerful oxidising agent.
(ii) Draw the structures of the following :
(1) XeF6
(2) IF7
Or
(b) (i) Explain why:
(1) Interhalogen compounds are more reactive than the related elemental halogens.
(2) Sulphur exhibits tendency for catenation but oxygen does not.
(3) On being slowly passed through water, PH3 forms bubbles but NH3 dissolves.
(ii) Complete and balance the following reactions:
(1) P4 + H2SO4 → ____ + _____ + _____ + _____
(2) Ag + HNO3(dilute) → _____ + ______ + _____ + _____
Answer:
(a) (i) F9 – 1s22s22p5
Cl17 – 1s22s22p63s23p5
(1) This is because in fluorine there is strong electron- electron repulsion for the incoming electron due to the relative compact size of 2p-orbitals in fluorine as compared to 3p-orbitals in chlorine.
(2) This is because of in red phosphorus, there are strong covalent bonds in P4 molecules but in white phosphorus, there are Van der waals forces of attraction in P4 molecules.
(3) This is because O3 molecules readily dissociates to give naseent oxygen which oxidises other substances.
O3 → O2 + [O] Nascent oxygen
(ii) (1) XeF6 has distorted octahedral structure
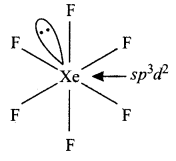
(2) IF7 has the pentagonal bipyramidal structure
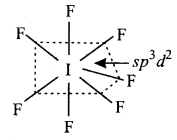
Or
(b) (i) (1) This is because these are polar and have lower bond energies.
(2) This is because of higher S—S bond energy \(\left(\frac{213 k \mathrm{J}}{\mathrm{mol}}\right)\) as compared to O-O bond energy \(\left(\frac{138 k \mathrm{J}}{\mathrm{mol}}\right)\)
(3) This is because PH3 is insoluble in water (HOH-bonds) but NH3 is highly soluble in water due to H-bonds.
(ii) (1) P4 + 10H2SO4 → 4H3PO4 + 10SO2 + 4H2O
(2) 3Ag + 4HNO3 → 3AgNO3 + NO + 2H2O
Question 18. [5]
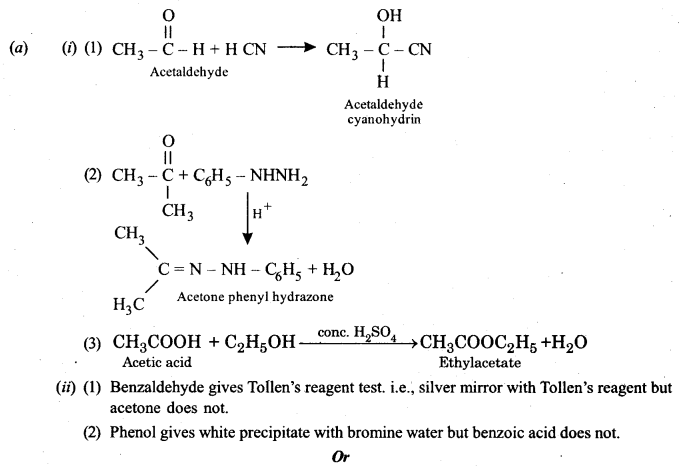
(a) (i) Give balanced chemical equations for the following reactions:
(1) Acetaldehyde reacts with hydrogen cyanide.
(2) Acetone reacts with phenylhydrazine.
(3) Acetic acid is treated with ethanol and a drop of conc. H2SO4.
(ii) Give one chemical test each to distinguish between the following pairs of compounds:
(1) Acetone and benzaldehyde.
(2) Phenol and benzoic acid.
Or
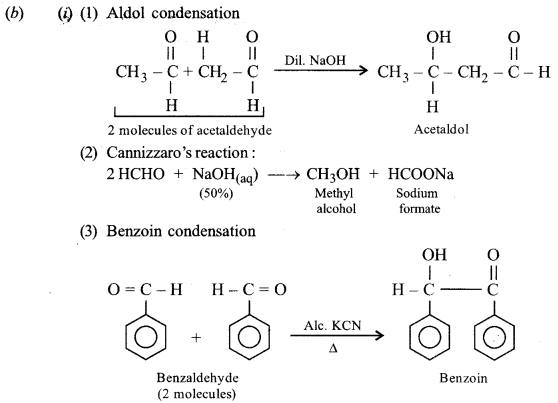
(b) (i) Write chemical equations to illustrate the following name reactions:
(1) Aldol condensation.
(2) Cannizzaro’s reaction.
(3) Benzoin condensation.
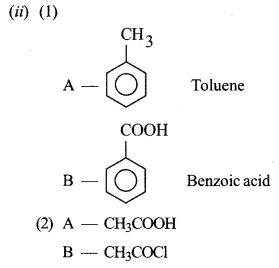
(ii) Identify the compounds A and B in the given reactions:
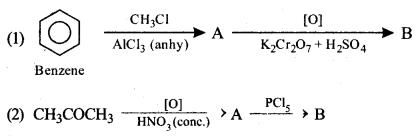
Answer: