ISC Chemistry Previous Year Question Paper 2017 Solved for Class 12
Maximum Marks: 70
Time allowed: 3 hours
- Answer all questions in Part I and six questions from Part II, choosing two questions from Section A, two from Section B and two from Section C.
- All working, including rough work, should be done on the same sheet as, and adjacent to, the rest of the answer.
- The intended marks for questions or parts of questions are given in brackets [ ].
- Balanced equations must be given wherever possible and diagrams where they are helpful.
- When solving numerical problems, all essential working must be shown.
- In working out problems use the following data:
Gas constant R = 1.987 cal deg-1 mol-1 = 8.314 JK-1 mol-1 = 0.0821 dm3 atm K-1 mol-1. 1L atm = 1 dm3 atm = 101.3 J. 1 Faraday = 96500 Coulombs.
Avogadro’s number = 6.023 × 1023
Part – I (20 Marks)
Answer all questions.
Question 1.
(a) Fill in the blanks by choosing the appropriate word/words from those given in the brackets: [5]
(iodoform, acetaldehyde, positive, greater, acidic, acetone, disaccharide, negative, increases, glucose, decreases, chloroform, polysaccharide, lactose, lesser, basic, cationic hydrolysis, anionic hydrolysis)
(i) Calcium acetate on heating gives ………. which gives ……… on heating with iodine and sodium hydroxide solution.
(ii) On dilution of a solution, its specific conductance ……….. while its equivalent conductance ……….
(iii) Sucrose is a ………… and yields upon hydrolysis, a mixture of ………. and fructose.
(iv) More ………. the standard reduction potential of a substance, the ……… is its ability to displace hydrogen from acids.
(v) An aqueous solution of CH3COONa is ………. due to …………
(b) Complete the following statements by selecting the correct alternative from the choices given: [5]
(i) In a face-centred cubic lattice, atom (A) occupies the comer positions and atom (B) occupies the face centre positions. If one atom of (B) is missing from one of the face-centred points, the formula of the compound is:
(1) A2B5
(2) A2B3
(3) AB2
(4) A2B
(ii) The half-life period of a first-order reaction is 20 minutes. The time required for the concentration of the reactant to change from 0.16 M to 0.02 M is:
(1) 80 minutes
(2) 60 minutes
(3) 40 minutes
(4) 20 minutes
(iii) For a spontaneous reaction ΔG° and E° cell will be respectively:
(1) -ve and +ve
(2) +ve and -ve
(3) +ve and +ve
(4) -ve and -ve
(iv) The conjugate acid of \(\mathrm{HPO}_{4}^{2-}\) is:
(1) H3PO3
(2) H3PO4
(3) \(\mathrm{H}_{2} \mathrm{PO}_{4}^{-}\)
(4) \(\mathrm{PO}_{4}^{3-}\)
(v) The polymer formed by the condensation of hexamethylenediamine and adipic acid is:
(1) Teflon
(2) Bakelite
(3) Dacron
(4) Nylon-66
(c) Answer the following questions: [5]
(i) Why the freezing point depression (ΔTf) of 0.4 M NaCl solution is nearly twice than that of 0.4 M glucose solution?
(ii) Identify the order of reaction from each of the following units of rate constant (k):
(a) mol L-1 sec-1
(b) mol-1 L sec-1
(iii) Specific conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm-1. Calculate its molar conductivity.
(iv) Name the order of reaction which proceeds with a uniform rate throughout.
(v) What are the products formed when phenol and nitrobenzene are treated separately with a mixture of concentrated sulphuric acid and concentrated nitric acid?
(d) Match the following: [5]
| (i) Diazotisation | (a) Bakelite |
| (ii) Argentite | (b) Nernst equation |
| (iii) Thermosetting plastics | (c) Aniline |
| (iv) Electrochemical cell | (d) Ethylenediamine |
| (v) Bidentate ligand | (e) Froth floatation process |
Answers:
(a) (i) acetone, iodoform
(ii) decreases, increases
(iii) disaccharide, glucose
(iv) negative, greater
(v) basic, anionic hydrolysis
(b) (i) (1)
(ii) (2)
(iii) (1)
(iv) (3)
(v) (4)
(c) (i) This is because Van’t Hoff factor for NaCl is 2 and for glucose, it is 1.
(ii) (a) – Zero
(b) – Two
\(\begin{aligned}(\text {iii}) &=\frac{k \times 1000}{\text { Molarity }} \\ &=\frac{0.025 \times 1000}{0.20} \\ \Lambda_{m} &=125 \mathrm{S} \mathrm{cm}^{2} \mathrm{mol}^{-1} \end{aligned}\)
(iv) Zero
(v) 2, 4, 6 – Trinitrophenol
m – Dinitrobenzene
(d) (i) – (c)
(ii) – (e)
(iii) – (a)
(iv) – (b)
(v) – (d)
Part – II (50 Marks)
Section – A
Answer any two questions.
Question 2.
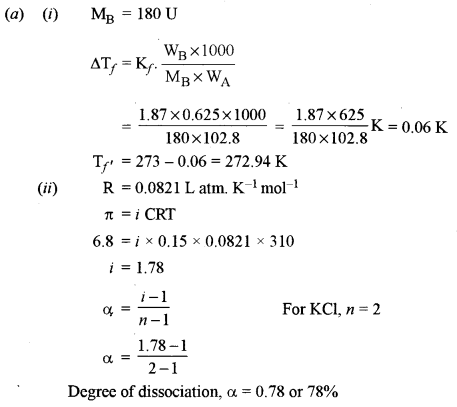
(a) (i) Determine the freezing point of a solution containing 0.625 g of glucose (C6H12O6) dissolved in 102.8 g of water. [2]
(Freezing point of water = 273 K, Kf for water = 1.87 K kg mol-1, at. wt. C = 12, H = 1, O = 16)
(ii) A 0.15 M aqueous solution of KCl exerts an osmotic pressure of 6.8 atm at 310 K. [2]
Calculate the degree of dissociation of KCl. (R = 0.0821 Lit. atm K-1 mol-1).
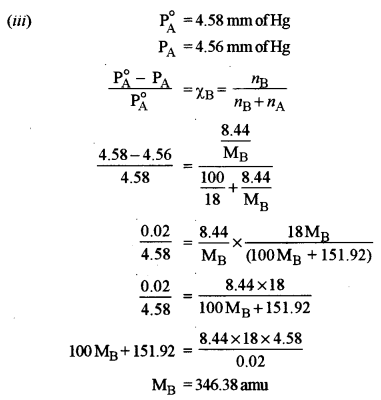
(iii) A solution containing 8.44 g of sucrose in 100 g of water has a vapour pressure 4.56 mm of Hg at 273 K. If the vapour pressure of pure water is 4.58 mm of Hg at the same temperature, calculate the molecular weight of sucrose. [1]
(b) (i) When ammonium chloride and ammonium hydroxide are added to a solution containing both Al3+ and Ca2+ ions, which ion is precipitated first and why? [2]
(ii) A solution of potassium chloride has no effect on litmus whereas, a solution of zinc chloride turns the blue litmus red. Give a reason. [2]
(c) How many sodium ions and chloride ions are present in a unit cell of sodium chloride crystal? [1]
Answer:
(b) (i) Al3+ ions are precipitated first as Al(OH)3 because Ksp for Al(OH)3 is lower than that of Ca(OH)2.
(ii) This is because KCl does not undergo hydrolysis in water and the aqueous solution is neutral whereas ZnCl2 undergoes hydrolysis in water to give an acidic solution which turns blue litmus solution red.
\(\begin{array}{rl}{\mathrm{Zn}^{2+}+2 \mathrm{C} \mathrm{I}^{-}+2 \mathrm{H}_{2} \mathrm{O}} & {\rightleftharpoons \mathrm{Zn}(\mathrm{OH})_{2}+2 \mathrm{H}^{+}+2 \mathrm{Cl}} \\ {\mathrm{or}} & {\mathrm{Zn}^{2+}+2 \mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{Zn}(\mathrm{OH})_{2}+2 \mathrm{H}^{+}}\end{array}\)
(c) Number of Na+ ions = 4
Number of Cl– ions = 4
Question 3.
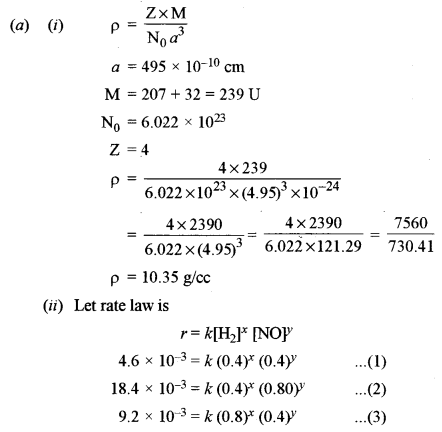
(a) (i) Lead sulphide has a face-centred cubic crystal structure. If the edge length of the unit cell of lead sulphide is 495 pm, calculate the density of the crystal. [1]
(at. wt. of Pb = 207, S = 32)
(ii) For the reaction: \(2 \mathrm{H}_{2}+2 \mathrm{NO} \rightleftharpoons 2 \mathrm{H}_{2} \mathrm{O}+\mathrm{N}_{2}\), the following rate data was obtained: [3]
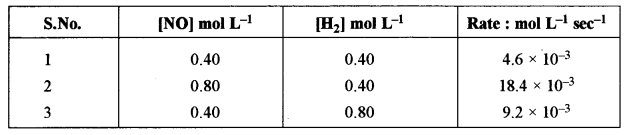
Calculate the following:
(1) The overall order of a reaction.
(2) The rate law.
(3) The value of rate constant (k).
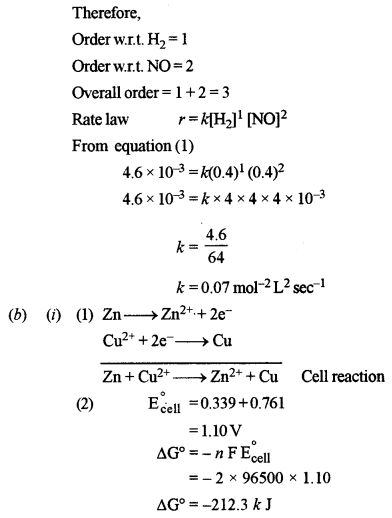
(b) (i) The following electrochemical cell is set up at 298 K: [2]
\(\begin{array}{c}{\mathrm{Zn} / \mathrm{Zn}^{2+}(\mathrm{aq})(1 \mathrm{M}) / / \mathrm{Cu}^{2+}(\mathrm{aq})(1 \mathrm{M}) / \mathrm{Cu}} \\ {\text { Given } \rightarrow \mathrm{E}_{Z n^{2+} / 2 \mathrm{n}}^{\circ}=-0.761 \mathrm{V}, \mathrm{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\circ}=+0.339 \mathrm{V}}\end{array}\)
(1) Write the cell reaction.
(2) Calculate the emf and free energy change at 298 K.
(a) Answer the following: [2]
(1) What is the effect of temperature on the ionic product of water (Kw)?
(2) What happens to the ionic product of water (Kw) if some acid is added to it?
(c) Frenkel defect does not change the density of the ionic crystal whereas, Schottky defect lowers the density of ionic crystal. Give a reason; [2]
Answer:
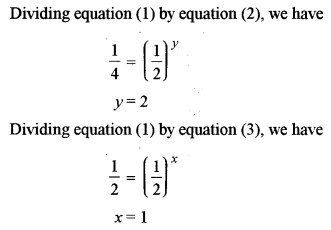
(ii) (1) Kw for water increases with the increase in temperature due to the increase in the degree of ionisation of water.
(2) Kw remains unchanged.
(c) This is because in Frenkel defect no ions are missing from the crystal lattice site whereas, in case of Schottky defect, an equal number of positive and negative ions are missing from the crystal lattice, hence density decreases.
Question 4.
(a) (i) Name the law or principle to which the following observations confirm: [3]
(1) When water is added to a 1.0 M aqueous solution of acetic acid, the number of hydrogen ion (H+) increases.
(2) When 9650 coulombs of electricity is passed through a solution of copper sulphate, 3.175 g of copper is deposited on the cathode (at. wt. of Cu = 63.5).
(3) When ammonium chloride is added to a solution of ammonium hydroxide, the concentration of hydroxyl ion decreases.
(ii) What is the difference between the order of a reaction and its molecularity? [2]
(b) (i) Explain why high pressure is required in the manufacture of sulphur trioxide by the contact process. State the law or principle used. [2]
(ii) Calculate the equilibrium constant (Kc) for the formation of NH3 in the following reaction: [ 1]
\(\mathrm{N}_{2}(\mathrm{g})+3 \mathrm{H}_{2}(\mathrm{g}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{g})\)
At equilibrium, the concentration of NH3, H2 and N2 are 1.2 × 10-2, 3.0 × 10-2 and 1.5 × 10-2 M respectively.
(c) Explain the following: [2]
(i) Hydrolysis of ester (ethyl acetate) begins slowly but becomes fast after some time.
(ii) The pH value of acetic acid increases on the addition of a few drops of sodium acetate.
Answers:
(a) (i) (1) This is due to the increase in the degree of ionisation of CH3COOH.
(2) W = ZQ
\(=\frac{63.5}{2 \times 96500} \times 9650\)
W = 3.175 g
(3) This is due to the common ion effect (NH4+ ions are common)
(ii)
| Order of a reaction | Molecularity |
| 1. It can be zero. 2. It can be the whole number or fractional. 3. It is defined for simple as well as complex reaction. 4. It is determined experimentally. | 1. It is never zero. 2. It is always a whole number. 3. It is defined only for simple reactions. 4. It is a theoretical concept. |
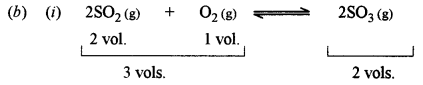
This is because the forward reaction is accompanied by a decrease in volume.
The principle used is i.e., Chatelier’s principle.
It states that when a system in equilibrium is subjected to stress, (i.e., change of concentration, temperature pressure etc.), the equilibrium tends to shift in a direction so as to undergo the effect of applied stress.
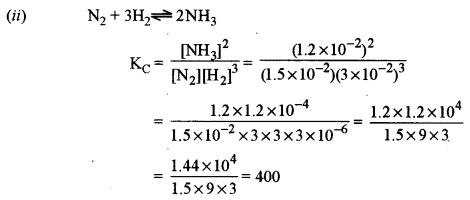
(c) (i) This is because of H+ ions produced during hydrolysis act as a catalyst.
(ii) This is due to the common ion effect. As a result of (H+) decreases.
Section – B
Answer any two questions.
Question 5.
(a) Write the formula of the following compounds: [2]
(i) Potassium trioxalatoaluminate (III).
(ii) Hexaaquairon (II) sulphate.
(b) Name the types of isomerism shown by the following pairs of compounds: [1]
(i) [CU(NH3)4] [PtCl4] and [Pt(NH3)4] [CuCl4]
(ii) [Co(Pn)2Cl2]+ and [Co(en)2Cl2]+
(c) For the coordination complex ion [Co(NH3)6]3+ [2]
(i) Give the IUPAC name of the complexion.
(ii) What is the oxidation number of cobalt in the complexion?
(iii) State the type of hybridisation of the complexion.
(iv) State the magnetic behaviour of the complexion.
Answers:
(a) (i) K3[Al(C2O4)3]
(ii) [Fe(H2O)6] SO4
(b) (i) Coordination isomerism
(ii) Linkage isomerism
(c) (i) hexaamminecobalt (II) ions
(ii) x + 6(0) = +3
x = +3
No. of Co in complex ion = +3
(iii) d2sp3
(iv) [CO(NH3)6]3+
Co3+ has no unpaired electron.
Hence, [Co(NH3)2]3+ is diamagnetic.
Question 6.
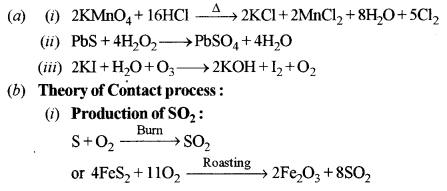
(a) Give balanced equations for the following reactions: [3]
(i) Potassium permanganate is heated with concentrated hydrochloric acid.
(ii) Lead sulphide is heated with hydrogen peroxide.
(iii) Ozone is treated with potassium iodide solution.
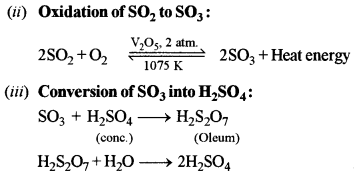
(b) Discuss the theory involved in the manufacture of sulphuric acid by the contact process. [2]
Answers:
Question 7.
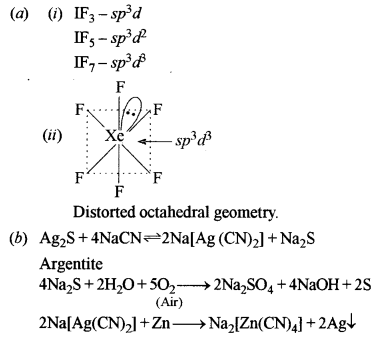
(a) (i) What are the types of hybridisation of iodine in interhalogen compounds IF3, IF5 and IF7, respectively? [3]
(ii) Draw the structure of xenon hexafluoride (XeF6) molecule and state the hybridisation of the central atom.
(b) Give the balanced equations for the conversion of argentite (Ag2S) to metallic silver. [2]
Answers:
Section – C
Answer any two questions.
Question 8.
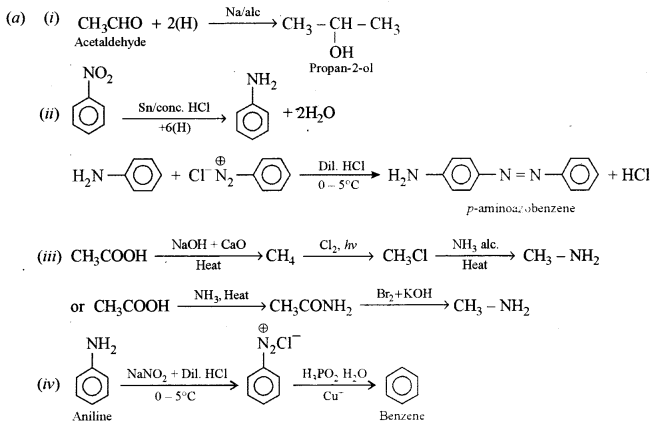
(a) How can the following conversions be brought about:
(i) Acetaldehyde to propan-2-ol. [1]
(ii) Nitrobenzene to p-aminoazobenzene. [1]
(iii) Acetic acid to methylamine. [2]
(iv) Aniline to benzene. [1]
(b) (i) How will you distinguish between primary, secondary and tertiary amines by Hinsberg’s test? [1]
(ii) Why do alcohols possess higher boiling points as compared to those of corresponding alkanes? [1]
(c) Identify the compounds A, B and C: [3]
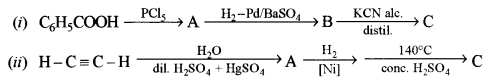
Answer:
(b) (i) With Hinsberg’s reagent:
Primary amines give N-alkyl benzene sulphonamide soluble in alkali.
Secondary amines give N, N-dialkyl benzene sulphonamide insoluble in alkali.
Tertiary amines have no action with Hinsberg’s reagent.
(ii) This is because in alcohols there are intermolecular H-bonds which are stronger than Van der Waals forces of attraction in alkanes.
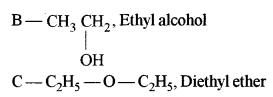
Question 9.
(a) Give balanced equations for the following name reactions: [3]
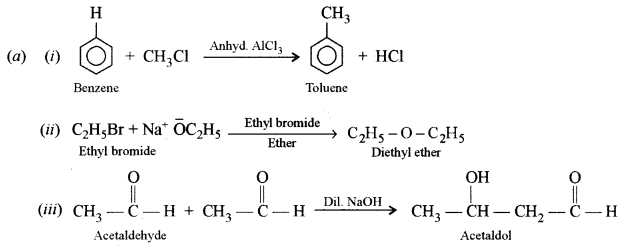
(i) Friedel-Crafts reaction (alkylation)
(ii) Williamson’s synthesis
(iii) Aldol condensation
(b) Give the chemical test to distinguish: [3]
(i) Ethyl alcohol and sec-propyl alcohol
(ii) Acetaldehyde and acetic acid
(c) (i) Deficiency of which vitamin causes the following diseases: [4]
(1) Scurvy
(2) Night blindness
(ii) Write two differences between globular and fibrous proteins.
Answers:
(b) (i) Victor Meyer’s test: Ethyl alcohol gives blood red colouration but sec-propyl alcohol gives deep blue colouration.
(ii) Acetic acid gives CO2 gas (effervescence) with NaHCO3 solution but acetaldehyde does not.
(c) (i) (1) Vitamin C
(2) Vitamin A
(ii)
| Globular proteins | Fibrous proteins |
| 1. They have spherical shapes. 2. These are soluble in water. 3. These are sensitive to small changes in temperature and pH. | 1. They have thread-like structures. 2. These are insoluble in water. 3. These are not affected by small change in temperature and pH. |
Question 10.
(a) An aliphatic unsaturated hydrocarbon (A) when treated with HgSO4/H2SO4 yields a compound (B) having molecular formula C3H6O. (B) on oxidation with concentrated HNO3 gives two compounds (C) and (D). Compound (C), when treated with PCl5, gives compound (E). (E) when reacts with ethanol gives a sweet-smelling liquid (F). Compound (F) is also formed when (C) reacts with ethanol in the presence of concentrated H2SO4. [4]
(i) Identify the compounds A, B, C, D, E and F.
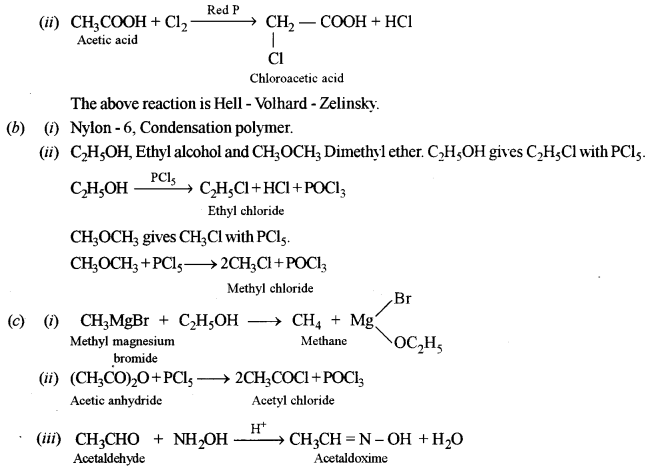
(ii) Give the chemical equation for the reaction of (C) with chlorine in the presence of red phosphorus and name the reaction.
(b) Answer the following: [3]
(i) What is the common name of the polymer obtained by the polymerisation of caprolactam? Is it addition polymer or condensation polymer?
(a) Name the two organic compounds which have the same molecular formula C2H6O. Will they react with PCl5? If they react, what are the products formed?
(c) Give balanced equations for the following reactions: [3]
(i) Methyl magnesium bromide with ethyl alcohol.
(ii) Acetic anhydride with phosphorus pentachloride.
(iii) Acetaldehyde with hydroxylamine.
Answer:
(a) (i) \(\mathrm{A}-\mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{CH}, \text { propyne }\)
B – CH3COCH3, Acetone
C – CH3COOH, Acetic acid
D – HCOOH, Formic acid (or CO2 + H2O)
E – CH3COCl, Acetyl chloride
F – CH3COOC2H5, Ethyl acetate