ICSE Chemistry Previous Year Question Paper 2013 Solved for Class 10
ICSE Paper 2013
CHEMISTRY
(Two Hours)
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the Question Paper.
The time given at the head of this Paper is the time allowed for writing the answers.
Section I is compulsory. Attempt any four questions from Section II.
The intended marks for questions or parts of questions are given in brackets [ ].
SECTION-I (40 Marks)
(Attempt all questions from this Section)
Question 1:
(a) From the list given below, select the word(s) required to correctly complete blanks (1) to (5) in the following passage. The words from the list are to be used only once. Write the answers as (a) (1), (2), (3) and so on. Do not copy the passage.
[ammonia, ammonium, carbonate, carbon dioxide, hydrogen, hydronium, hydroxide, precipitate, salt water]:
A solution M turns blue litmus red, so it must contain (1) …………. ions; another solution O turns red litmus blue and hence, must contain (2) …………. ions. When solutions M and O are mixed together, the products will be (3) ………… and (4) ………… If a piece of magnesium was put into a solution M, (5) ………. gas would be evolved. [5]
(b) Identify the gas evolved in the following reactions when:
- Sodium propionate is heated with soda lime.
- Potassium suphite is treated with dilute hydrochloric acid.
- Sulphur is treated with concentrated nitric acid.
- A few crystals of KNO3 are heated in a hard glass test tube.
- Concentrated hydrochloric acid is made to react with manganese dioxide. [5]
(c) State one appropriate observation for each of the following:
- Concentrated sulphuric acid is added drop wise to a crystal of hydrated capper sulphate.
- Copper sulphide is treated with dilute hydrochloric acid.
- Excess of chlorine gas is reacted with ammonia gas.
- A few drops of dilute hydrochloric acid are added to silver nitrate solution, followed by addition of ammonium hydroxide solution.
- Electricity is passed through molten lead bromide. [5]
(d) Give suitable chemical terms for the following:
- A bond formed by a shared pair of electrons with both electrons coming from the same atom.
- A salt formed by incomplete neutralisation of an acid by a base.
- A reaction in which hydrogen of an alkane is replaced by a halogen.
- A definite number of water molecules bound to some salts.
- The process in which a substance absorbs moisture from the atmospheric air to become moist, and ultimately dissolves in the absorbed water. [5]
(e) Give a chemical test to distinguish between the following pairs of compounds: [5]
- Sodium chloride solution and sodium nitrate solution.
- Hydrogen chloride gas and hydrogen sulphide gas.
- Ethene gas and ethane gas.
- Calcium nitrate solution and zinc nitrate solution.
- Carbon dioxide gas and sulphur dioxide gas.
(f) Choose the most appropriate answer from the following options: [10]
- Among the period 2 elements, the element which has high electron affinity is:
(A) Lithium (B) Carbon
(C) Chlorine (D) Fluorine - Among the following compounds identify the compound that has all three bonds (ionic, covalent and coordinate bond)
(A) Ammonia (B) Ammonium chloride
(C) Sodium hydroxide (D) Calcium chloride - Identify the statement that is incorrect about alkanes:
(A) They are hydrocarbons.
(B) There is a single covalent bond between carbon and hydrogen.
(C) They can undergo both substitution as well as addition reactions.
(D) On complete combustion they produce carbon dioxide and water. - Which of these will act as a non-electrolyte?
(A) Liquid carbon tetrachloride
(B) Acetic acid
(C) Sodium hydroxide aqueous solution acid
(D) Potassium chloride aqueous solution - Which one of the following will not produce an acid when made to react with water?
(A) Carbon monoxide (B) Carbon dioxide
(C) Nitrogen dioxide (D) Sulphur trioxide - Identify the metallic oxide which is amphoteric in nature:
(A) Calcium Oxide (B) Barium oxide
(C) Zinc oxide (D) Copper(II) oxide - In the given equation identify the role played by concentrated sulphuric acid
S + 2H2SO4 → 3SO2 + 2H2O:
(A) Non-volatile acid (B) Oxidising agent
(C) Dehydrating agent (D) None of the above - Nitrogen gas can be obtained by heating:
(A) Ammonium nitrate (B) Ammonium nitrite
(C) Magnesium nitride (D) Ammonium chloride - Which of the following is not a typical property of an ionic compound?
(A) High melting point.
(B) Conducts electricity in the molten and in the aqueous solution state.
(C) They are insoluble in water.
(D) They exist as oppositely charged ions even, in the solid state. - The metals zinc and tin are present in the alloy:
(A) Solder (B) Brass
(C) Bronze (D) Duralumin
(g) Solve the following:
(i) What volume of oxygen is required to burn completely 90 dm3 of butane under similar conditions of temperature and pressure? [2]
2C4H10 + 13O2 → 8CO2 + 10H2O
(ii) The vapour density of a gas is 8. What would be the volume occupied by 24.0g of the gas at STP? [2]
(iii) A vessel contains X number of molecules of hydrogen gas at a certain temperature and pressure. How many molecules of nitrogen gas would be present in the same vessel under the same conditions of temperature and pressure? [1]
Answer:
(a)
- Hydronium
- Hydroxide
- Salt
- Water
- Hydrogen
(b)
- Ethane (C2H6)
- Sulphur dioxide (SO2)
- Nitrogen dioxide (NO2)
- Oxygen (O2)
- Chlorine (Cl2)
(c)
- The blue colour of the solution changes to white.
- A gas evolved which has the smell of rotten eggs.
- A yellow coloured explosive is formed.
- Curdy white ppt. observed which is soluble in NH4OH.
- Reddish brown vapours of bromine are evolved.
(d)
- Co-ordinate bond
- Acidic salt
- Halogenation
- Water of crystallisation
- Deliquescence
(e)
- Sodium chloride gives white ppt. with silver nitrate solution while sodium nitrate does not.
- Dense white fumes observed when a rod dipped in NH3 is brought to the mouth of t.t containing HCl gas. Whereas no such fumes observed in case of H2S gas.
- Ethene gas decolorises the purple colour of KMnO4 whereas no change observed with ethane.
- Add NaOH solution to both the solutions. White ppt. observed with Zn(NO3)2 solution which is soluble in excess. Whereas white ppt. observed with Ca(N03)2 which is sparingly soluble in water.
- SO2 turns acidified potassium dichromate solution green whereas CO2 does not change the colour.
(f)
- D
- B
- C
- A
- A
- C
- B
- C
- C
- C
(g)
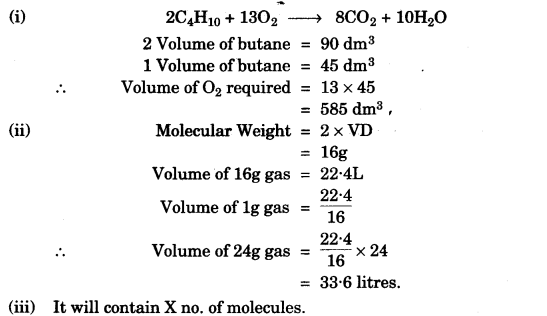
SECTION-II (40 Marks)
(Answer any four questions from this section)
Question 2:
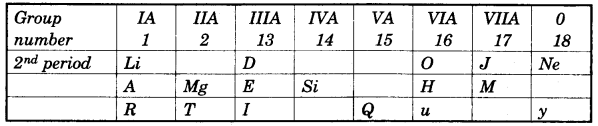
In this table H does not represent hydrogen. Some elements are given in their own symbol and position in the periodic table. While others are shown with a letter.
With reference to the table answer the following questions:
- Identify the most electronegative element. [1]
- Identify the most reactive element of group 1. [1]
- Identify the element from period 3 with least atomic size. [1]
- How many valence electrons are present in Q? [1]
- Which element from group 2 would have the least ionization energy? [1]
- Identify the noble gas of the fourth period. [1]
- In the compound between A and B what type of bond would be formed and give the molecular formula for the same. [2]
(b) Compare the compounds carbon tetrachloride and sodium chloride with regard to solubility in water and electrical conductivity. [2]
Answer:
(a)
- J
- R
- M
- 5 electron
- T
- y
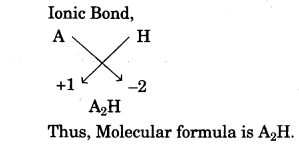
(b) Carbon tetrachloride is insoluble in water and behave as a bad conductor of electricity while sodium chloride is soluble in water and behave as a good conductor of electricity in their aqueous state.
Question 3:
(a) Choosing the substances from the list given belou), write balanced chemical equations for the reactions which would be used in the laboratory to obtain the following salts:

(b) State two relevant observations for each of the following:
- Ammonium hydroxide solution is added to copper(II) nitrate solution in small quantities and then in excess.
- Ammonium hydroxide solution is added to zinc nitrate solution in minimum quantities and then in excess.
- Lead nitrate crystals are heated in a hard glass test tube. [6]
Answer:
(a)
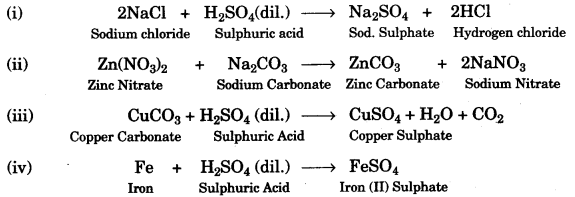
(b)
- (a) When NH4OH is added to copper (II) nitrate solution in small quantities then a pale blue ppt. is observed.
(b) When added in excess it disolves to give an inky blue solution forming a complex salt. - (a) When added in small quantities is forms a gelatinous white ppt.
(b) When added in excess it dissolves to form a complex salt. - (a) Reddish brown gas is evolved.
(b) Colourless, odourless gas evolved which rekindles a glowing splinter.
Question 4:
(a) Copper sulphate solution is electrolysed using copper electrodes.
Study the diagram given below and answer the question that follows:
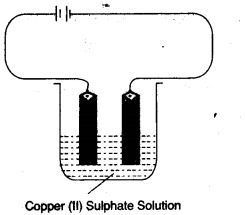
- Which electrode to your left or right is known as the oxidising electrode and why? [2]
- Write the equation representing the reaction that occurs. [1]
- State two appropriate observations for the above electrolysis reaction. [2]
(b)
| X | Y | |
| Normal Electronic Configuration | 2, 8, 7 | 2, 8, 2 |
| Nature of oxide | Dissolves in water and turns blue litmus red. | Very low solubility in water. Dissolves in hydrochloric acid. |
| Tendency for oxidizing and reducing reactions | Tends to oxidise elements and compounds. | Tends to act as a reducing agent. |
| Electrical and Thermal conductivity | Very poor electrical conductor. Poor thermal conductivity. | Good Electrical conductor. Good Thermal conductor. |
| Tendency to form alloys and amalgams | No tendency to form alloys | Forms alloysUsing the information above, complete the following: |
Using the information above, complete the following:
- ………. is the metallic element.
- Metal atoms tend to have a maximum of ………… electrons in the outermost energy level.
- Non-metallic elements tend to form ……………. oxides while metals tend to form ………….. oxides.
- Non-metallic elements tend to be ………….. conductors of heat and electricity.
- Metals tend to …………….. electrons and act as ………….. agents in their reactions with elements and compounds. [5]
Answer:
(a)
- Right electrode is known as oxidising electrode because copper ion gain electron to form copper metal i.e., reduction takes place.
- At Catode: Cu2+ + 2e– → Cu
At Anode: Cu – 2e– → Cu2+ - (a) Reddish brown metal copper is deposited at cathode.
(b) Blue colour of electrolytic solution i.e., CuSO4 does not fades during the process.
(b)
- Y
- 2
- Acidic, Basic
- Bad
- lose, reducing
Question 5:
(a) Give balanced equations for each of the following:
(i) Reduction of hot Copper(II) oxide to copper using ammonia gas.
(ii) Oxidation of carbon with concentrated nitric acid.
(iii) Dehydration of concentrated sulphuric acid with sugar crystals. [3]
(b) Copy and complete the following table relating to important industrial process: [3]
| Name of the process | Temperature | Catalyst | Equation for the catalyzed reaction |
| Haber’s process |
(c) The following questions relate to the extraction of aluminium by electrolysis:
- Name the other aluminium containing compound added to alumina and state its significance.
- Give the equation for the reaction that takes place at the cathode.
- Explain why is it necessary to renew the anode periodically. [4]
Answer:
(a)
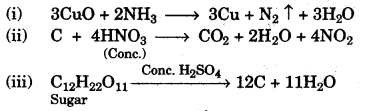
(b) Temperature: 450 – 500 0C
Catalyst: Finely divided iron.
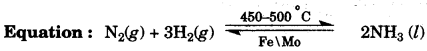
(c)
- Cryolite (Na3AlF6)
It reduces the temperature and enhance conductivity. - At Cathode: Al3+ + 3e– → Al
- The anode has got to be replaced periodically, as it gets oxidised by the oxygen evolved at the anode.
Question 6:
(a) Give balanced equations for the laboratory preparations of the following organic compounds:
(i) A saturated hydrocarbon from iodomethane.
(ii) An unsaturated hydrocarbon from an alcohol.
(iii) An unsaturated hydrocarbon from calcium carbide.
(iv) An alcohol from ethyl bromide. [4]
(b) Give the structural formulae for the following:
(i) An isomer of n-butane.
(ii) 2-propanol.
(iii) Diethyl ether. [3]
(c) Give reasons for the following:
- Methane does not undergo addition reactions, but ethene does.
- Ethyne is more reactive than ethane.
- Hydrocarbons are excellent fuels. [3]
Answer:
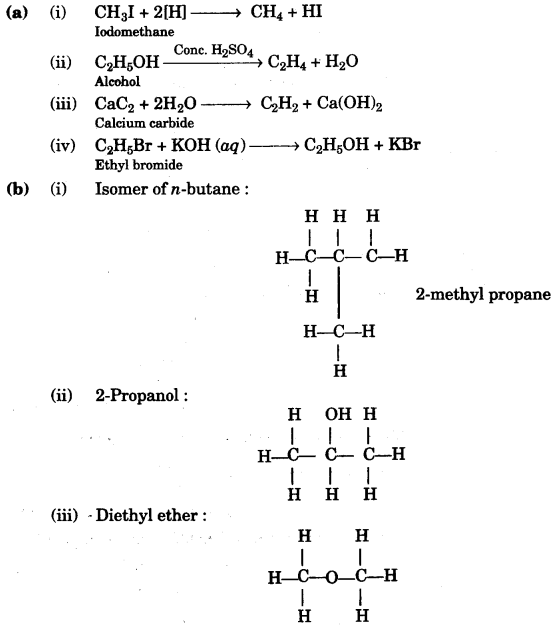
(c)
- Methane does not undergo addition reaction, because it is bonded with four H-atom while in ethene double bond breaks and provide site for addition.
- Due to the presence of triple bond it provide site for addition, hence ethyne is more reactive than ethane.
- Hydrocarbon are excellent fuels as they produces lot of heat during combustion.
Question 7:
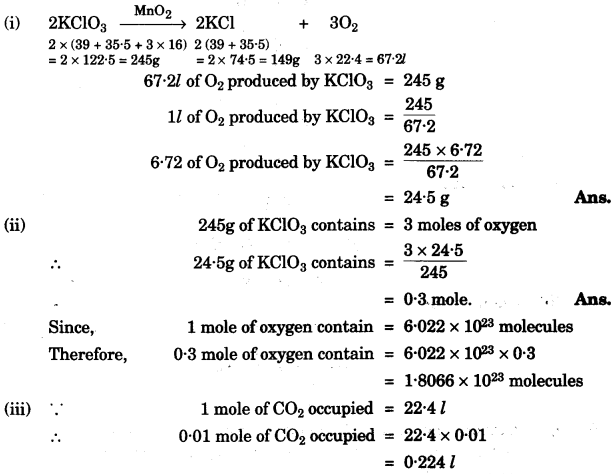
(a) O2 is evolved by heating KClO3 using MnO2 as a catalyst.
![]()
(i) Calculate the mass of KClO3 required to produpe 6.72 litre of 02 at STP.
[atomic masses of K = 39, Cl = 35.5, O = 16] [2]
(ii) Calculate the number of moles of oxygen present in the above volume and also the number of molecules. [2]
(iii) Calculate the volume occupied by 0.01 mole of CO2 at STP. [1]
(b) Identify the following substances which are underlined:
- An alkaline gas which produces dense white fumes when reacted with hydrogen chloride gas.
- An acid which is present in vinegar.
- A gas which does not conduct electricity in the liquid state but conducts electricity when dissolved in water.
- A dilute mineral acid which forms a white precipitate when treated with barium chloride solution.
- The element which has the highest ionization potential. [5]
Answer:
(a)
(b)
- Ammonia gas (NH3)
- Acetic Acid (CH3COOH)
- Hydrogen chloride (HCl)
- Sulphuric Acid (H2SO4)
- Helium (He)
