ICSE Chemistry Previous Year Question Paper 2012 Solved for Class 10
ICSE Paper 2012
CHEMISTRY
(Two Hours)
Answers to this Paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the Question Paper.
The time given at the head of this Paper is the time allowed for writing the answers.
Section I is compulsory. Attempt any four questions from Section II.
The intended marks for questions or parts of questions are given in brackets [ ].
SECTION-I (40 Marks)
(Attempt all questions from this Section)
Question 1:
(a) Name the gas in each of the following:
- The gas evolved on reaction of Aluminium with boiling concentrated caustic alkali solution.
- The gas produced when excess ammonia reacts with chlorine.
- A gas which turns acidified potassium dichromate clear green.
- The gas produced when copper reacts with concentrated nitric acid.
- The gas produced on reaction of dilute sulphuric acid with a metallic sulphide: [5]
(b) State one observation for each of the following:
- Excess ammonium hydroxide solution is added to lead nitrate solution.
- Bromine vapours are passed into a solution of ethyne in carbon tetrachloride.
- A zinc granule is added to copper sulphate solution.
- Zinc nitrate crystals are strongly heated.
- Sodium hydroxide solution is added to ferric chloride solution at first a little and then in excess. [5]
(c) Some word I words are missing in the following statements. You are required to rewrite the statements in the correct form using the appropriate word/words:
- Ethyl alcohol is dehydrated by sulphuric acid at a temperature of about 170°C.
- Aqua regia contains one part by volume of nitric acid and three parts by volume of hydrochloric acid.
- Magnesium nitride reacts with water to liberate ammonia.
- Cations migrate during electrolysis.
- Magnesium reacts with nitric acid to liberate hydrogen gas. [5]
(d) Choose the correct answer from the options given below: [5]
- An element in period-3 whose electron affinity is zero.
(A) Neon (B) Sulphur
(C) Sodium (D) Argon - An alkaline earth metal.
(A) Potassium (B) Calcium
(C) Lead (D) Copper - The vapour density of carbon dioxide [C = 12, O = 16].
(A) 32 (B) 16
(C) 44 (D) 22 - Identify the weak electrolyte from the following:
(A) Sodium Chloride solution (B) Dilute Hydrochloric acid
(C) Dilute Sulphuric acid (D) Aqueous acetic acid - Which of the following metallic oxides cannpt be reduced by normal reducing agents?
(A) Magnesium oxide (B) Copper(II) oxide
(C) Zinc oxide (D) Iron(II) oxide
(e) Match the following: [5]
| Column A | Column B |
| 1. Acid salt | A. Ferrous ammonium Sulphate |
| 2. Double salt | B. Contains only ions |
| 3. Ammonium hydroxide solution | C. Sodium hydrogen sulphate |
| 4. Dilute hydrochloric acid | D. Contains only molecules |
| 5. Carbon tetrachloride | E. Contains ions and molecules |
(f) Give the structural formula for the following:
(i) Methanoic acid
(ii) Ethanal
(iii) Ethyne
(iv) Acetone
(v) 2-methyl propane [5]
(g) Concentrated nitric acid oxidises phosphorus to phosphoric acid according to the following equation:
P + 5HNO3 (conc.) → H3PO4 + H2O + 5NO2
If 9.3g of phosphorus was used in the reaction, calculate:
(i) Number of moles of phosphorus taken. [1]
(ii) The mass of phosphoric acid formed. [2]
(iii) The volume of nitrogen dioxide produced at STP.
[H = 1, N = 14, P = 31, O = 16] [2]
(h) Give reasons for the following:
- Iron is rendered passive with fuming nitric acid.
- An aqueous solution of sodium chloride conducts electricity.
- Ionisation potential of the element increases across a period.
- Alkali metals are good reducing agents.
- ydrogen chloride gas cannot be dried over quick lime. [5]
Answer:
(a)
- Hydrogen
- Nitrogen
- Sulphur dioxide
- Nitrogen dioxide
- Hydrogen sulphide
(b)
- Insoluble chalky white ppt of lead hydroxide is obtained.
- Reddish brown colour of bromine water disappears.
- The blue colour of copper sulphate solution disappears.
- Reddish brown fumes of nitrogen dioxide a colourless gas that rekindles a glowing splint are released. In the test tube, a ppt is left which is yellow when hot and white when cold.
- Insoluble reddish brown ppt obtained.
(c)
- Ethyl alcohol is dehydrated by concentrated sulphuric acid at a temperature of about 170°C.
- Aqua regia contains one part by volume of concentrated nitric acid and three parts by volume of concentrated hydrochloric acid.
- Magnesium nitride reacts with boiling water to liberate ammonia.
- Cations migrate to cathode dining electrolysis.
- Magnesium reacts with very dilute nitric acid to liberate hydrogen gas.
(d)
- D-Argon
- B-Calcium
- D-22
- D-Aqueous acetic acid
- A-Magnesium oxide
(e)
| Column A | Column B |
| 1. Acid salt | C. Sodium hydrogen sulphate |
| 2. Double salt | A. Ferrous ammonium Sulphate |
| 3. Ammonium hydroxide solution | E. Contains ions and molecules |
| 4. Dilute hydrochloric acid | B. Contains only ions |
| 5. Carbon tetrachloride | D. Contains only molecules |
(f)
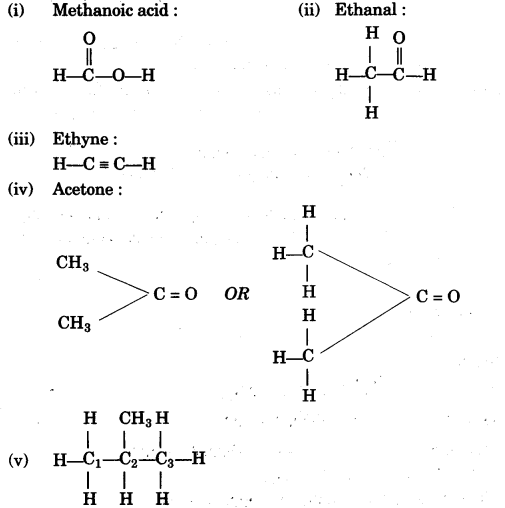
(g)
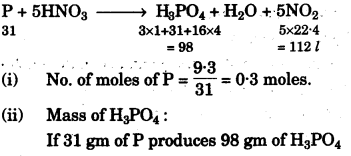
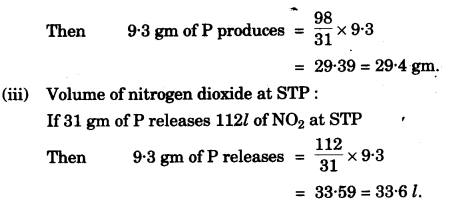
(h)
- Cone. HNO3 being a strong oxidising agent oxidises iron, forming a layer that makes iron non reactive or passive.
- Aqueous solution of sodium chloride contains mobile ions like Na+, Cl–, H+, OH–, H3O+ etc. so they conduct electricity.
- Atomic size decreases and nuclear charges increases as we move from left to right in a period so energy required to remove one electron from the valence shell increases from left to right thus ionisation potential increases.
- Alkali metals readily lose electrons from their valence shell and get oxidised. So they behave as good reducing agents.
- Hydrogen chloride is acidic whereas quick lime is basic. So they will react with each other hence quick lime can not be used to dry hydrogen chloride.
SECTION-II (40 marks)
(Answer any four questions from this section)
Question 2:
(a) Some properties of sulphuric acid are listed below. Choose the role played by sulphuric acid as A, B, C or D which is responsible for the reactions (i) to (v). Some role/s may be repeated. [5]
A. Dilute acid.
B. Dehydrating agent.
C. Non-volatile acid
D. Oxidising agent
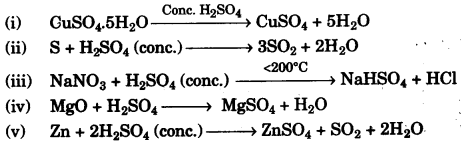
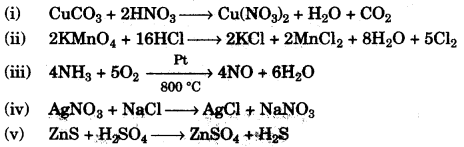
(b) Give balanced equations for the following reactions: [5]
(i) Dilute nitric acid and Copper carbonate.
(ii) Concentrated hydrochloric acid and Potassium permanganate solution.
(iii) Ammonia and Oxygen in the presence of a catalyst.
(iv) Silver nitrate solution and Sodium chloride solution.
(v) Zinc sulphide and Dilute sulphuric acid.
Answer:
(a)
(i) B – Dehydrating agent.
(ii) D – Oxidising agent
(iii) C – Non-volatile acid
(iv) A – Dilute acid.
(v) D – Oxidising agent
(b)
Question 3:
(a) Select, the correct answer from the list given in brackets:
- An aqueous electrolyte consists of the ions mentioned in the list, the ion which could be discharged most readily during electrolysis.
[Fe2+, Cu2+, Pb2+, H+] - The metallic electrode which does pot take part in an electrolytic reaction.
[Cu, Ag, Pt, Ni]. - The ion which is discharged at the anode during the electrolysis of copper Usulphate solutions using copper electrodes as anode and cathode.
[Cu2+, OH–, SO42-, H+] - When dilute sodium chloride i$ electrolysed using graphite electrodes, the cation is discharged at the catode most readily.
[Na+, OH–, H+, Cl–] - During silver plating of an article using potassium argentocyanide as an electrolyte, the anode material should be [Cu, Ag, Pt, Fe]. [5]
(b) Match the properties and uses of alloys in List 1 with the appropriate answer from List 2. [5]
| List 1 | List 2 |
| 1. The alloy, contains Cu and is hard, silvery and is used in decorative articles. | A. Duralumin |
| 2. It is stronger than Aluminium, light and is used in making light tools. | B. Brass |
| 3. It is lustrous, hard, corrosion resistant and used in surgical instruments. | C. Bronze |
| 4. Tin lowers the melting point of the alloy and is used for soldering purpose. | D. Stainless steel |
| 5. The alloy is hard, brittle, takes up polish and is | E. Solder |
Answer:
(a)
- Cu2+
- Pt
- Cu2+/Nil
- Na+
- Ag
(b)
- (B) Brass
- (A) Duralumin
- (D) Solder
- (D) Stainless steel
- (C) Bronze
Question 4:
(a) Identify the anion present in the following compounds:
- Compound X on heating with copper turnings and.concentrated sulphuric acid liberates a reddish brown gas.
- When a solution of compound Y is treated with silver nitrate solution a white precipitate is obtained which is soluble in excess of ammonium hydroxide solution.
- Compound Z which on reacting with dilute sulphuric acid liberates a gas which turns lime water milky, but the gas has no effect on acidified potassium dichromate solution.
- Compoud L on reacting with Barium chloride solution gives a white precipitate insoluble in dilute hydrochloric acid or dilute nitric acid. [4]
(b) State one chemical test between each of the following pairs:
- Sodium carbonate and Sodium sulphite.
- Ferrous nitrate and Lead nitrate.
- Manganese dioxide and Copper(II) oxide. [3]
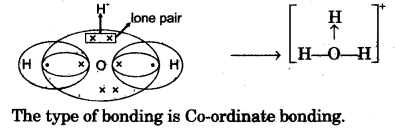
(c) Draw an electron dot diagram to show the structure of hydronium ion. State the type of bonding present in it. [3]
Answer:
(a)
- NO3– (Nitrate ion)
- Cl– (Chloride ion)
- CO3– – (Carbonate ion)
- SO4– – (Sulphate ion)
(b)
| 1. Test | Sodium Carbonate | Sodium Sulphite |
| Add dil. HCl or dil. H2SO4 | Colourless, odourless gas that turn lime water milky but has no effect on acidified potassium dichromate solution is released i.e., CO2 | Colourless gas with smell of burning sulphur, turn lime water milky and also turn acidified potassium dichromate green is released i.e., SO2 gas. |
| 2. Test | Ferrous Nitrate | Lead Nitrate |
| Add few drops of NaOH | Dirty green ppt of Ferrous hydroxide | Chalky white ppt of lead hydroxide. |
| 3. Test | Manganese dioxide | Copper (II) oxide |
| Heat with cone. HCl | Greenish yellow gas with irritating smell and acidic nature is released i.e., chlorine gas. | No reaction. |
(c)
Question 5:
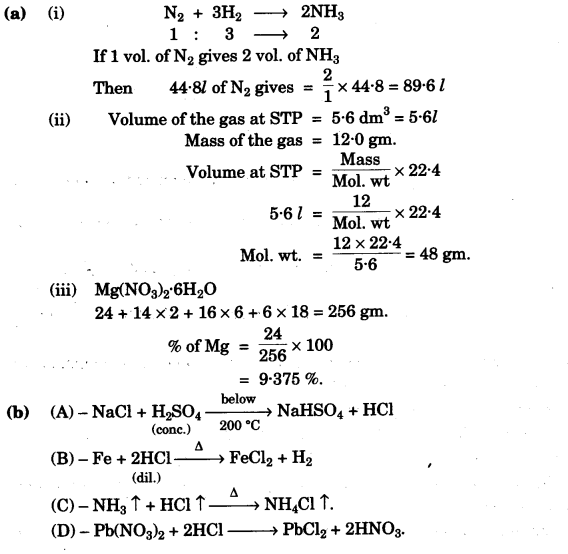
(a) (i) 67.2 litres of hydrogen combines with 44.8 litres of nitrogen to form ammonia under specific conditions as:
N2(g) + 3H2(g) → 2NH3(g)
Calculate the volume of ammonia produced. ,What is the other substance, if any, that remains in the resultant mixture? [2]
(ii) The mass of 5.6 dm3 of a certain gas at STP is 12.0 g. Calculate the relative molecular mass of the gas. [2]
(iii) Find the total percentage of Magnesium in magnesium nitrate crystals,
Mg(NO3)2.6H20. [Mg = 24; N = 14; 0 = 16 and H = 1] [2]
(b) Refer to the flow chart diagram below and give balanced equations with conditions, if any, for the following conversions A to D. [4]
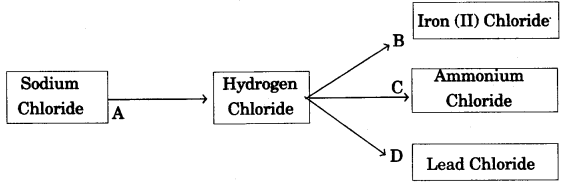
Answer:
Question 6:
(a) Name the following metals:
- A metal present in cryolite other than sodium.
- A metal which is unaffected by dilute or concentrated acids.
- A metal present in period 3, group 1 of the peribdic table. [3]
(b) The following questions are relevant to the extraction of Aluminium:
(i) State the reason for addition of caustic alkali to bauxite ore during purification of bauxite.
(ii) Give a balanced chemical equation for the above reaction.
(iii) Along with cryolite and alumina, another substance is added to the electrolyte mixture. Name the substance and give one reason for the addition. [3]
(c) The following questions are based on the preparation of ammonia gas in the laboratory:
- Explain why ammonium nitrate is not used in the preparation of ammonia.
- Name the compound normally used as a drying agent during the process.
- How is ammonia gas collected?
- Explain why it is not collected over water. [4]
Answer:
(a)
- Aluminium
- Gold
- Sodium
(b) (i) To dissolve bauxite ore and obtain a solution of Sodium Aluminate.
![]()
(iii) Fluorspar/CaF2.
To reduce the high melting point of alumina and to make it a conducting medium.
(c)
- Ammonium nitrate is a highly explosive substance and can not be heated.
- Quicklime/CaO.
- By downward displacement of air or upward delivery as it is lighter than air.
- Ammonia is highly soluble in water so it cannot be collected over water.
Question 7:
(a) From the following organic compounds given below, choose one compound in each case which relates to the description [i] to [iv]:
[Ethyne, ethanol, acetic acid, ethene, methane]
- An unsaturated hydrocarbon used for welding purposes.
- An organic compound whose functional group is carboxyl.
- A hydrocarbon which on catalytic hydrogenation gives a saturated hydrocarbon.
- An organic compound used as a thermometric liquid. [4]
(b)
(i) Why is pure acetic acid known as glacial acetic acid?
(ii) Give a chemical equation for the reaction between ethyl alcohol and acetic acid. [2]
(c) There are three elements E, F, G with atomic numbers 19, 8, and 17 respectively.
(i) Classify the elements as metals and non-metals. [3]
(ii) Give the molecular formula of the compound formed between E and G and
state the type of chemical bond in this compound. [1]
Answer:
(a)
- Ethyne
- Acetic acid
- Ethene
- Ethanol
(b) (i) Pure acetic acid on cooling forms an ice like mass so it is called glacial acetic acid.
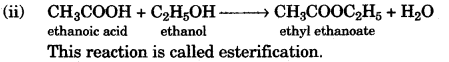
(c) E = 19 = 2, 8, 8, 1
F = 8 = 2, 6
G = 17 = 2, 8, 7
(i) E = Metal, F & G = Non metal
![]()
