How ceramics are made?
Ceramics:
- Ceramics are made from clay and composed of aluminium silicate mixed with sand.
- The white clay used to make ceramics is kaolin which is rich in kaolinite or hydrated aluminosilicate, Al2.O32SiO2.2H20.
- Red clay consists of iron(III) oxide which gives the red colour. Bricks, tiles, mugs and clay pots are some examples of traditional ceramics.
- During the making of ceramics, the shaped objects are heated to a very high temperature; they undergo a series of chemical reactions and are hardened to form ceramics.
- These chemical reactions are irreversible and the ceramics cannot be melted and remoulded.
How are ceramics made?
- Wet clay can be shaped easily because the tiny crystals in it can slide over each other. Clay has a plastic property. When the clay dries up, it keeps its shape as the crystals are now stuck together.
- When heated to above 1500°C, a series of chemical reactions produce other chemicals and glass which packs the tiny mineral crystals together.
- The object is now glazed and heated again. The reactions in the glaze cause the surface to be waterproof.
Properties and Uses of Ceramics
- The general properties of ceramic are as follows:
- Very hard and strong
- Brittle
- Chemically inert and does not corrode
- Good insulator of electricity and heat
- Very high melting point and heat resistance
- Porous but can be made impervious by glazing
- The properties of ceramics make them very useful in our daily life. Table shows the properties and uses of ceramics.
Property Uses Examples Hard and strong Buiiding
materialsTiles, bricks, roofs, cement, abrasive for grinding Attractive, easily moulded and glazed Decorative pieces and household items Vases, porcelain ware, sinks, bathtubs Chemically inert and non-corrosive Kitchenware Cooking pots, plates, bowls Very high melting point and good insulator of heat Insulation Lining of furnace, engine parts Electrical insulators Insulating parts in electrical appliances Spark plugs, insulators in ovens and electric cables Inert and non-compressible Medical and dental apparatus Artificial teeth and bones
- The need for high performance materials has helped us to speed up the development of ceramic science and engineering.
- Unlike glass, ceramic can withstand high temperatures and does not melt easily.
- Below are some common properties of glass and ceramic:
- Brittle
- Insulators of heat
- Hard and will not bend
- Good electrical insulators
- Good heat insulators
- Strong under compression
- Cannot withstand being stretched
- Inert to chemicals
- Do not corrode
- There are three important differences between glass and ceramic.
(a) Glass can be heated until molten repeatedly but not ceramic.
(b) Glass is usually transparent, ceramic is not.
(c) Glass has a lower melting point than ceramic.
People also ask
- How is glass produced from raw materials?
- How is a composite material made?
- How polymers are classified?
- What is alloy metal?
Improvement on the properties of glass and ceramic
- Continuous research and development has been done towards the development of glass and ceramic with improved qualities and specific purpose.
- The following are some improved glass: glass optical fibre, smart glass, photochromic glass and conducting glass.
- Example of new uses of improved ceramics are bioceramics, glass-ceramics, ceramic superconductors and ceramic composites.
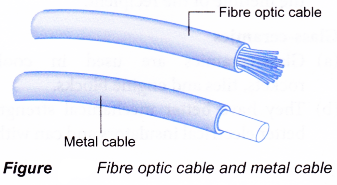
- Glass optical fibre
(a) A glass optical fibre is a pure glass thread that can transmit messages modulated onto light waves. It consists of a bundle of glass or plastic threads.
(b) Glass optical fibre is used in telephone
companies, control board displays and television, local-area networks and in medical instruments.
(c) Advantages of glass optical fibres are as follows:- Messages can be transmitted digitally rather than analogically
- Data can be transmitted over long distances without distortion and lost of signal
- Much thinner and lighter than the traditional cables
- Photochromic glass
(a) Photochromic glass contains photosensitive chemical such as silver chloride, silver bromide or copper(I) chloride that changes colour when exposed to ultraviolet light, and becomes clear again when the light intensity decreases.
When light intensity increases:
2AgBr → 2Ag + Br2
When light intensity decreases:
2Ag + Br2 → 2AgBr
(b) Photochromic glass is used for windows, sunglasses and instrument controls. - Conducting glass
(a) Conducting glass contains a thin layer of indium tin(IV) oxide embedded into glass, enabling the glass to conduct electricity. This glass is also known as ITO glass which is used in liquid crystal display (LCD) panels.
(b) Another type of conducting glass contains gold fibres embedded into glass, enabling it to conduct electric current to produce heat. It is used in front glass panel of the windscreen of an aircraft. At a very high altitude, the drop in temperature may cause the windscreen to be foggy. The current that flows through the glass can heat up the glass to improve visibility. - Smart glass
(a) Example of smart glass are electrochromic glass and privacy glass.
(b) Electrochromic glass is able to change its transmissivity upon receiving an electric signal.
(c) A privacy glass is a milky opaque glass that sandwiches a layer of liquid. It becomes clear when current flows through it. - Bioceramics
(a) Bioceramics are used as replacement parts such as bones, hip and joints in the human body.
(b) These bioceramics will last longer throughout the lifetime of the recipient. - Glass-ceramics
(a) Glass-ceramics are used in cookware, rockets, tiles and engine blocks.
(b) They have better mechanical strength, are better electrical insulators and can withstand a very high temperature without melting compared to normal glass.
(c) The use of this ceramic in engine block can reduce pollution and loss of energy. This is because fuel can be burnt at a very high temperature providing a more complete combustion. - Ceramic superconductors
(a) Perovskites, YBa2Cu3O7, is an example of ceramic that contains lanthanum, yttrium or bismuth or thallium, barium or strontium, copper and oxygen that can conduct electricity at 95°C. Superconductors are materials that can conduct electricity with zero resistance, thus preventing loss of energy in the form of heat.
(b) Ceramic superconductors are used to make very powerful and light magnets which are more powerful and lighter than normal magnets. - Ceramic composites
(a) Ceramic composites are made by combining ceramics with other materials such as metals, plastics or fibres.
(b) Ceramic composites are used to make high temperature furnaces, nuclear reactors and space shuttles.
(c) Piezoelectric ceramic is used in the controls for helicopter rotors to vary the pitch angle so as to twist the blade upwards and downwards. This ceramic can also reduce the weight by 8% and cut drag by 26%. - In years to come, scientists and engineers will develop more smart materials for our building industries through constant research and development.
