History of Aluminium
Symbol | Al | Atomic number | 13 |
History
Aluminium was first extracted in 1827 from aluminium chloride by treating it with sodium :
AlCl3 + 3Na → Al + 3NaCl.
But the process was very expensive. Impressed by its properties, metallurgists all over the world attempted to develop a process for the commercial production of aluminium. But it remained a costly metal till 1886, when Heroult in France and Hall in USA succeeded in developing independently a process for the extraction of aluminium. Thus began large-scale production of aluminium in several parts of the world. Hall-Heroult process came into extensive use towards the end of the nineteenth century.
Electrolytic reduction of alumina: Hall-Heroult process
Alumina is mixed with cryolite and the mixture is melted in an iron cell.
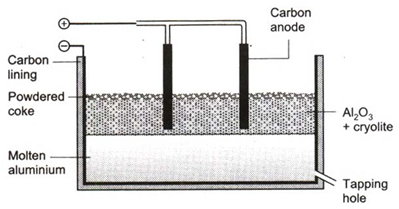 Alumina melts at 2303 K. It is a bad conductor of electricity. But when mixed with cryolite and some calcium fluoride, the mixture becomes a good conductor of electricity, and melts at 1173-1223 K. The cryolite thus considerably reduces the’ energy cost. The iron cell is lined inside with gas carbon, which serves as cathode. Carbon rods act as anode. The electrolyte, thus, contains Na+, Al3+, F– and O2– ions.
Alumina melts at 2303 K. It is a bad conductor of electricity. But when mixed with cryolite and some calcium fluoride, the mixture becomes a good conductor of electricity, and melts at 1173-1223 K. The cryolite thus considerably reduces the’ energy cost. The iron cell is lined inside with gas carbon, which serves as cathode. Carbon rods act as anode. The electrolyte, thus, contains Na+, Al3+, F– and O2– ions.
On passing electric current, AP+ ions are discharged at the cathode and the 022ions, at the anode.
Al3+ + 3e → Al (at cathode)
2O2– → O2 + 4e (at anode)
Some quantity of oxygen formed in the reaction escapes and some reacts with the anode to form CO2, Thus, carbon anode burns away due to its reaction with oxygen.
C + O2 → CO2
Hence, the anode has to be replaced from time to time. This increases the cost of production of aluminium. Molten aluminium sinks to the bottom, and is taken out from there.
Alloy | Composition | Uses |
1. Brass | Cu = 80%, Zn = 20% | Harder than pure Cu and Zn ; used for making utensils, cartridges, etc. |
2. Bronze | Cu = 90%, Sn = 10% | For making statues, medals, ships, coins, machines, etc. |
3. Solder (common) | Sn = 50%, Pb = 50% | For joining metals, soldering wires, electronic components, etc. |
4. Duralumin | Al = 95.5%, Cu = 3% Mn = 1% Mg = 0.5% | In bodies of aircraft, kitchenware, automobile parts, etc |
5. Babbit metal | Sn = 90%, Sb = 7%, Cu = 3% | In antifriction lining |
6. German silver | Cu = 60%, Zn = 20%, Ni = 20% | For making utensils, ornaments, etc. |
7. Gun metal | Cu = 60%, Sn = 10% | Gears, castings, etc. |
8. Bell metal | Cu = 78%, Sn = 22% | Bells, gongs, etc. |
9. Magnalium | Al = 90%, Mg = 10% | Balance beams, light instruments, etc. |
| 10. Pewter | Sn = 75%, Pb = 25% | Cups, mugs, etc. |
| 11. Type metal | Pb=82%, Sb = 15%, Sn = 3% | Casting type |
Alloy steels | |||
Name | Composition | Properties | Uses |
1. Manganese | Mn = 10 – 18% | Extremely hard, resistant to wear | Grinding machines, safes, etc. |
2. Chrome-vanadium | Cr = 1-10%, V = 0.15% | Highly tensile, resistance to stress and torsin | Axle and other parts of automobiles |
3. Nickel-chromium | Ni = 1-4%, Cr = 0.5-2% | High tensile strength, hard and highly elastic | Armour plates |
4. 18–8 | Cr = 18%, Ni = 8% | Resistance to corrosion | Cutlery, instrument |
| 5. Alnico | Co = 5% | Highly magnetic | Powerful permanent magnet |
