What is heat of precipitation?
Heat of precipitation:
- When two aqueous solutions are added together and a precipitate is formed, this reaction is called a precipitation reaction or double decomposition. This reaction is used to prepare insoluble salts.
- The heat given out in a precipitation reaction is called the heat of precipitation.
- The heat of precipitation is the heat change when one mole of a precipitate is formed from its ions in aqueous solution under standard conditions.
Some examples of precipitation reactions are:
- When barium chloride solution is added to sodium sulphate solution, a white precipitate, barium sulphate, is formed.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
Ba2+(aq) + SO42-(aq) → BaSO4(s) - When silver nitrate solution is added to hydrochloric acid, a white precipitate, silver chloride, is formed.
AgNO3(aq) + HCl(aq) → AgCl(s) + HNO3(aq)
Ag+(aq) + Cl–(aq) → AgCl(s) - When lead(II) nitrate solution is added to potassium iodide solution, a yellow precipitate, lead(II) iodide, is formed.
Pb(NO3)2(aq) + 2KI(aq) → PbI2 + 2KNO3(aq)
Pb2+(aq) + 2I–(aq) → PbI2(s)
People also ask
- How can energy be changed in a chemical reaction?
- How does the energy level diagram show this reaction is exothermic?
- What is enthalpy of reaction?
- What is heat of displacement?
- What is the enthalpy of neutralization?
- What is the heat of combustion?
- Why is energy released when a bond is formed?
- Applications of exothermic and endothermic reactions in everyday life
- What is meant by the calorific value of a fuel?
Heat of precipitation of silver chloride experiment
Aim: To determine the heat of precipitation of silver chloride.
Materials: 0.5 mol dm-3 silver nitrate solution, 0.5 mol dm-3 sodium chloride solution.
Apparatus: Thermometer, plastic cups with covers, 50 cm3 measuring cylinders.
Caution:
Add the two solutions together as quickly as possible.
Stir the mixture throughout the activity.
Safety measures:
Handle the chemicals with caution. Wear eye protection.
Silver nitrate solution may cause dark stains on your skin and clothing.
Procedure:
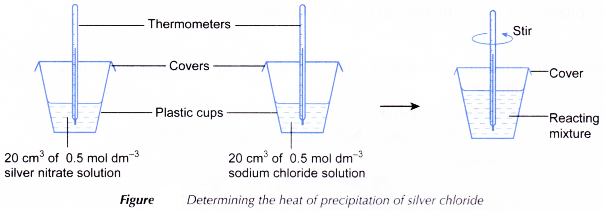
- 20 cm3 of 0.5 mol dm-3 silver nitrate solution is measured and poured into a plastic cup.
- The initial temperature of the silver nitrate solution is measured after a few minutes.
- 20 cm3 of 0.5 mol dm-3 sodium chloride solution is measured.
- The initial temperature of the sodium chloride solution is measured after a few minutes.
- The sodium chloride solution is added quickly and carefully to the silver nitrate solution. The mixture is stirred with the thermometer.
- The highest temperature of the reacting mixture is measured and recorded.
Results:
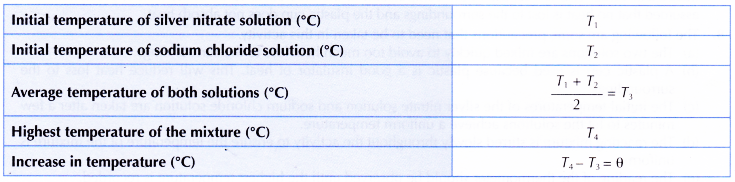
Interpreting data:
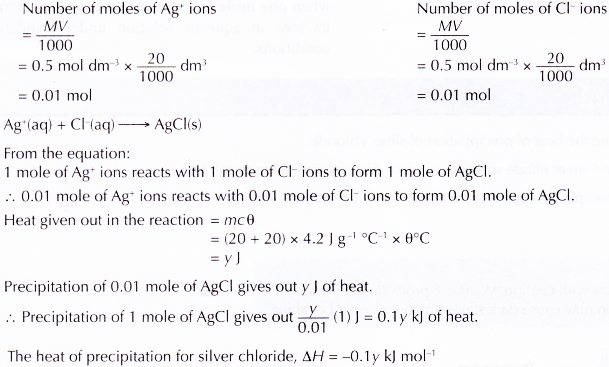
Discussion:
- When silver nitrate solution and sodium chloride solution are added, a white precipitate is formed. The precipitate is silver chloride.
- The chemical equation for the reaction is:
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) - The ionic equation for the reaction is:
Ag+(aq) + Cl–(aq) → AgCl(s) - The thermochemical equation for the precipitation of silver chloride is:
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) ΔH = -0.1 y kJ mol-1
or
Ag+(aq) + Cl–(aq) → AgCl(s) ΔH = -0.1 y kJ mol-1 - The energy level diagram for the precipitation of silver chloride is:

- The theoretical value for the heat of precipitation for silver chloride is -65.5 kJ mol-1.
- The value for the heat of precipitation of silver chloride obtained from this activity is always less than the theoretical value. This is because in the computation of the theoretical value of the heat of precipitation, it is assumed that no heat is lost to the surroundings and the plastic cup does not absorb heat.
- The following are some precautions that need to be taken in this activity.
(a) The two solutions are mixed quickly to avoid too much heat loss to the surroundings.
(b) A plastic cup is used because plastic is a good insulator of heat. This will reduce heat loss to the surroundings.
(c) The initial temperatures of the silver nitrate solution and sodium chloride solution are taken after a few minutes to let the solutions achieve a uniform temperature.
(d) The reacting mixture is stirred slowly throughout the activity to ensure the temperature of the mixture is uniform.
(e) The reading of the thermometer should be observed until the highest temperature is recorded. - If the activity is repeated using twice the volume of silver nitrate solution and sodium chloride solution, but of the same concentration, the change in temperature will be the same. This is because:
(a) When the volumes of both reactants are doubled, the number of moles of both reactants is also doubled. This makes the heat change double, 2H.
(b) The mass of the solution is also doubled, 2m.
(c) Heat change, H = mcθ
When the volumes of both reactants are doubled, heat change, 2H = 2mcθ.
Cancelling the number 2 from both sides of the equation gives H = mcθ.
Thus, θ remains the same.
If the sodium chloride solution is replaced with hydrochloric acid of the same concentration, the heat of precipitation is still the same. This is because both sodium chloride solution and hydrochloric acid provide the same amount of chloride ions. Sodium ions, Na+ and hydrogen ions, H+ are spectator ions. The reaction involves Ag+ ions and Cl– ions.
Conclusion:
The heat of precipitation for silver chloride is -0.1 y kJ mol-1.
How to calculate heat of precipitation example problems with solutions
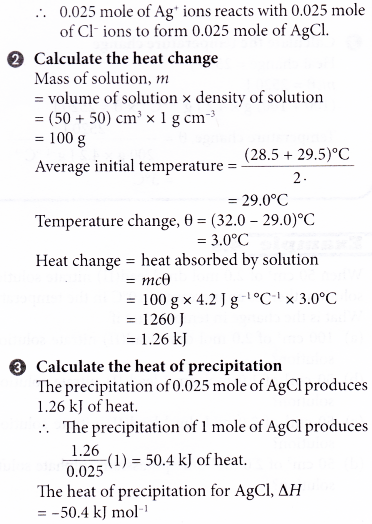
1. 50 cm3 of 0.5 mol dm-3 silver nitrate solution at 29.5°C is added to 50 cm3 of 0.5 mol dm-3 potassium chloride solution which is at a temperature of 28.5°C. The mixture is stirred and the highest temperature reached is 32.0°C. Calculate the heat of precipitation for silver chloride.
[Specific heat capacity of solution: 4.2 J g-1 °C-1. Density of solution: 1 g cm-3]
Solution:
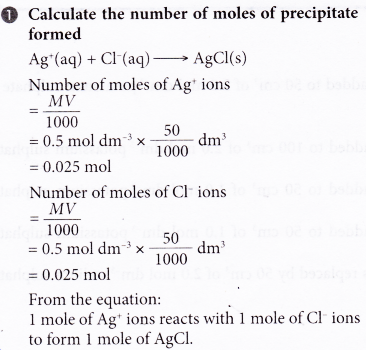
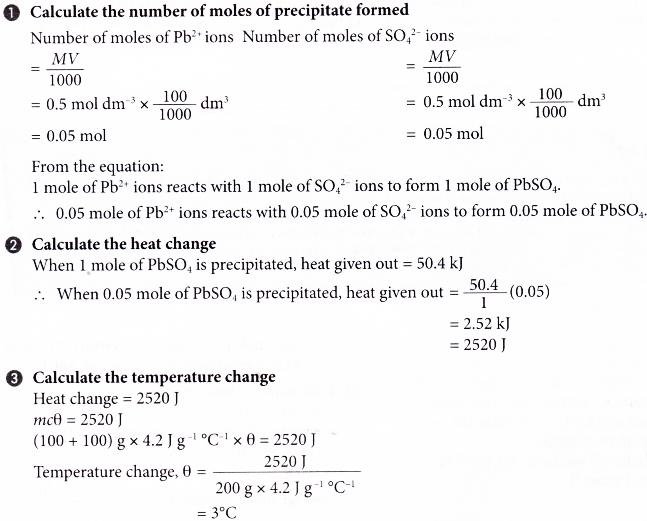
2. The thermochemical equation for the precipitation of lead(II) sulphate is given below.
Pb2+(aq) + SO42-(aq) → PbSO4(s) ΔH = -50.4 kJ
When 100 cm3 of 0.5 mol dm-3 lead(II) nitrate solution is added to 100 cm3 of 0.5 mol dm-3 sodium sulphate solution, lead(II) sulphate is precipitated. What is the temperature change in the reacting mixture in the experiment?
[Specific heat capacity of solution = 4.2 J g-1 °C-1. Density of solution = 1 g cm-3]
Solution:
3. When 50 cm3 of 2.0 mol dm-3 lead(II) nitrate solution is added to 50 cm3 of 2.0 mol dm-3 potassium sulphate solution, there is an increase of 10°C in the temperature.
What is the change in temperature if
(a) 100 cm3 of 2.0 mol dm-3 lead(II) nitrate solution is added to 100 cm3 of 2.0 mol dm-3 potassium sulphate solution?
(b) 50 cm3 of 1.0 mol dm-3 lead(II) nitrate solution is added to 50 cm3 of 1.0 mol dm-3 potassium sulphate solution?
(c) 50 cm3 of 2.0 mol dm-3 lead(II) nitrate solution is added to 50 cm3 of 1.0 mol dm-3 potassium sulphate solution?
(d) 50 cm3 of 2.0 mol dm-3 potassium sulphate solution is replaced by 50 cm3 of 2.0 mol dm-3 sodium sulphate solution?
Solution:
(a) The volumes of both reactants are doubled.
By calculation:
When 50 cm3 of 2.0 mol dm-3 of lead(II) nitrate solution and 50 cm3 of 2.0 mol dm-3 potassium sulphate solution are used,

When 100 cm3 of 2.0 mol dm-3 of lead(II) nitrate solution and 100 cm3 of 2.0 mol dm-3 potassium sulphate solution are used,
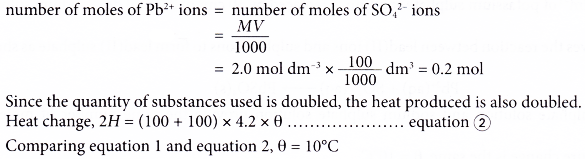
By deduction:
When the volumes of both reactants are doubled, the number of moles of both reactants is also doubled. This makes the heat change double, 2H.
The mass of the solution is also doubled, 2m.
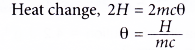
(b) The concentration of both reactants are halved.
By calculation:
When 50 cm3 of 1.0 mol dm-3 lead(II) nitrate solution and 50 cm3 of 1.0 mol dnr3potassium sulphate solution are used,
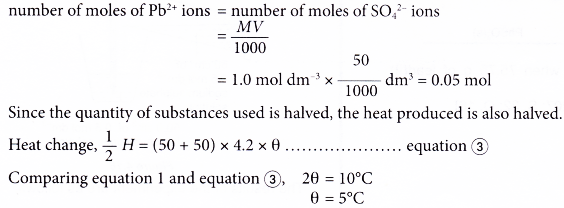
By deduction:
When the concentrations of both reactants are halved, the number of moles of both reactants is also halved.
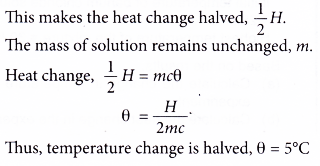
(c) The concentration of potassium sulphate solution is halved. When 50 cm3 of 2.0 mol dm-3 lead(II) nitrate solution and 50 cm3 of 1.0 mol dm-3 potassium sulphate solution are used,

Potassium sulphate is the limiting substance, thus the calculation is based on 0.05 mole of SO42- ions.
Since the number of moles of substances is halved, the heat produced is also halved.
Mass of solution is the same, thus
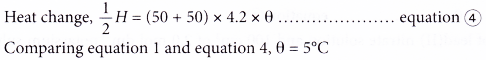
(d) 50 cm3 of 2.0 mol dm-3 of potassium sulphate solution is replaced by 50 cm3 of 2.0 mol dm-3 sodium sulphate solution.
This reaction involves the reaction between lead(II) ions and sulphate ions to form lead(II) sulphate as shown below.
Pb2+(aq) + SO42-(aq) → PbSO4(s)
Both potassium sulphate solution and sodium sulphate solution provide the same number of moles of sulphate ions.
Thus, the temperature change is the same, θ = 10°C
