What is Heat Capacity?
Heat Capacity
On a sunny morning, a worker at a swimming pool uses his hand to feel the water in the swimming pool and in a pail. The water in the pail is warm but the water in the swimming pool is still cold.
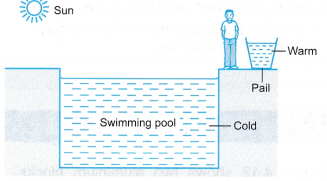 (a) Both the water in the pail and the water in the swimming pool absorb heat energy from the sun.
(a) Both the water in the pail and the water in the swimming pool absorb heat energy from the sun.
(b) The mass of water in the swimming pool is greater than the mass of water in the pail.
(c) The water in the swimming pool needs to absorb more heat before it warms up.
(d) The water in the swimming pool is said to have higher heat capacity than the water in the pail.
- (a) The heat capacity of an object is the amount of heat required to increase its temperature by 1°C.
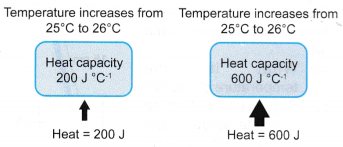
(b) Heat capacity is measured in J °C-1 or J K-1.Object with larger heat capacity Object with smaller heat capacity Needs to absorb more heat to raise its temperature by 1 °C. Needs to absorb less heat to raise its temperature by 1 °C. Releases more heat when it cools down by 1 °C. Releases less heat when it cools down by 1 °C. - The concept of heat capacity is illustrated in Figure.
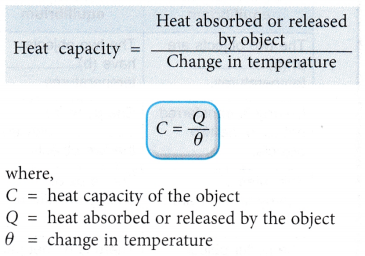
- The heat capacity of an object is calculated in the following way:
- The heat capacity of an object depends on the
(a) mass of the object
(b) type of material - An object with a larger mass has a larger heat capacity than an object with a smaller mass of the same material.
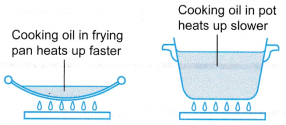
- (a) The cooking oil in the frying pan has a smaller heat capacity than the cooking oil in the pot.
(b) Heat is supplied at the same rate to both the cooking oil in the frying pan and in the pot.
(c) The cooking oil in the frying pan heats up faster than the cooking oil in the pot.
People also ask
- What is the Formula for Specific Heat Capacity?
- What is meant by Latent Heat?
- What is the latent heat of fusion of Ice?
- What is latent heat of vaporization of Water?
Heat Capacity Experiments
1. Aim: To observe the change in temperature when the same amount of heat is used to heat different masses of water.
Material: Tap water
Apparatus: Three sets of the following: 600 ml glass beaker, 200 W immersion heater, thermometer, weighing scale, retort stand with clamp
Method:
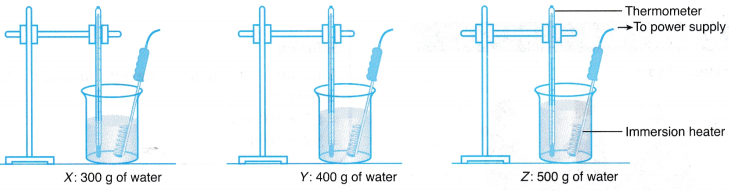
- Three beakers, X, Y and Z, are filled with 300 g, 400 g and 500 g of tap water respectively.
- The apparatus is set up as shown in Figure.
- The initial temperature of the water in beaker X is recorded in Table.
- The immersion heater is switched on.
- The temperature of the water in beaker X is recorded every 0.5 minutes for 5 minutes.
- Steps 3 to 5 are repeated for the water in beakers Y and Z.
- The graphs of temperature against time for all three beakers of water are plotted on the same set of axes.
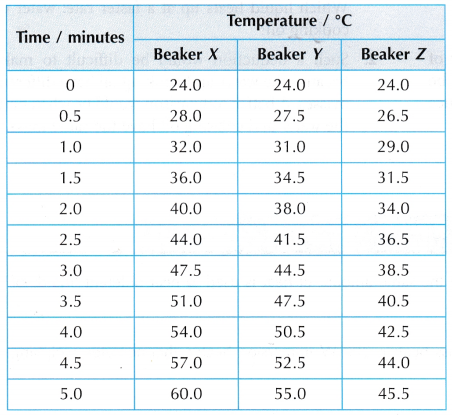
Results:
Discussion:
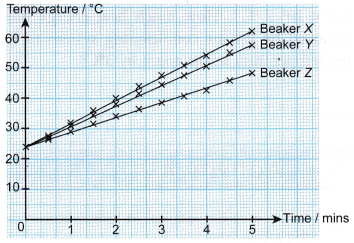
- The graphs of temperature against time have different gradients. Therefore, the temperature of the water in the three beakers increases at different rates.
- The rate of increase of the temperature of the water in the beakers is: X > Y > Z.
- The heat capacities of the water in the beakers is: X < Y < Z.
- The mass of water in the beakers is: X < Y < Z.
- The larger the mass of the water, the larger is its heat capacity.
2. Aim: To observe the change in temperature when the same amount of heat is used to heat different liquids of the same mass.
Materials: Tap water, cooking oil
Apparatus: Two sets of the following: 600 ml glass beaker, 200 W immersion heater, thermometer, weighing scale, retort stand with clamp
Method:
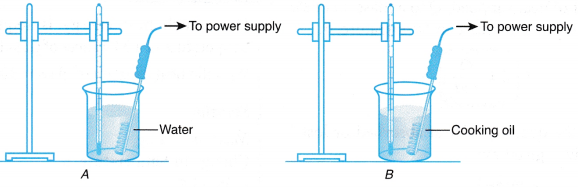
- Two beakers, A and 6, are each filled with 400 g of tap water and cooking oil respectively.
- The apparatus is set up as shown in Figure.
- The initial temperature of the water in beaker A is recorded in Table.
- The immersion heater is switched on.
- After 5 minutes, the heater is switched off. The reading of the thermometer is observed and the highest reading is recorded as the final temperature of the water.
- Steps 3 to 5 are repeated for beaker B containing cooking oil.
Results:

Discussion:
- The water and cooking oil have equal masses.
- Both the heaters have the same power rating and were switched on for the same period of time. Therefore, the same amount of heat was supplied to each liquid.
- The increase in temperature of water is less than the increase in temperature of cooking oil.
- This means that water requires more heat to increase its temperature by 1°C.
- Therefore, water has a higher specific heat capacity than cooking oil. 5
Heat Capacity Example Problems with Solutions
Example 1. A block of aluminium has a heat capacity of 180 J °C-1. Calculate the amount of heat required to raise its temperature from 30°C to 35°C.
Solution:
Heat capacity = 180 J °C-1
Heat required to increase the temperature by 1°C is 180 J.
Increase in temperature = 35°C – 30°C = 5°C
Heat required to increase the temperature by 5°C
= 180 x 5
= 900 J
Example 2. The air in a meeting room has a heat capacity of 1.3 x 105 J °C-1. How much heat energy must be removed by the air conditioning system to reduce the temperature in the room by 3°C?
Solution:
Amount of heat energy needed to be removed
= 1.3 x 105 x 3
= 3.9 x 105 J
