General Properties of Salts
Some of the characteristic properties of salts are:
- Melting and boiling points: Salts are mostly solids which melt as well as boil at high temperatures.
- Solubility in water: Salts are generally soluble in water. For example, sodium chloride, potassium sulphate, aluminium nitrate, ammonium carbonate, etc., are soluble salts while silver chloride, lead chloride, copper carbonate, etc., are insoluble in water.
- Water of crystallization: Generally, salts are found as crystals with water molecules present in them. This water is called water of crystallization and such salts are called hydrated salts.
For example, copper sulphate crystal has five molecules of water for each copper sulphate molecule. This is written as CuSO4.5H2O. This water of crystallization gives the crystal its shape. It also gives colour to some crystals. On heating, hydrated salts lose their water of crystallization and, as a result, the crystals lose their shape and colour and change to a powdery substance.
The hydrated salts that have lost their water of crystallization are called anhydrous salts.
When hydrated copper sulphate is heated, it gives out water molecules to form white powdery anhydrous copper sulphate. On addition of water, this substance can convert back to a hydrated copper sulphate solution again.
People also ask
- Classification of Salts
- Uses of different salts in daily life
- Preparation of Salts
- Describe the preparation of soluble and insoluble salts
- Qualitative Analysis of Salts
- Action of Heat on Salts
- Test for Cations and Anions in Aqueous Solutions
- Constructing ionic equations using the continuous variation method
- What is stoichiometry and why is it used in chemistry?
General Properties of Salts :
1. Reaction with an acid : When a salt reacts with an acid, another salt and acid are formed. For example, when sodium chloride is heated with sulphuric acid, sodium hydrogensulphate (at low temperature) and then sodium sulphate (at high temperature) are produced and hydrogen chloride gas is evolved.
2. Reaction with a base : A salt reacts with a base to produce another salt and base.
(NH4)2SO4 + 2NaOH → Na2SO4 + 2NH4OH
3. Reaction with a metal : Sometimes, a salt solution may react with a metal. For example, when an iron nail is dipped into an aqueous solution of copper sulphate, copper gets deposited on the surface of the nail and the ferrous sulphate formed remains in the solution.
CuSO4 + Fe → FeSO4 + Cu
This reaction shows that iron is more reactive than copper.
Thus, more reactive metal can displace a less reactive metal from a solution of its salt.
4. Behaviour of salts towards water :
When a salt is dissolved in water, the solution may be neutral, acidic or alkaline. This depends upon the nature of the salt used.
(i) A normal salt derived from a strong acid and a strong base gives a neutral solution. For example, the aqueous solutions of NaCl and K2SO4 are neutral to litmus.
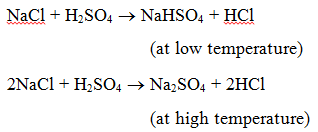
(ii) A normal salt derived from a weak acid and a strong base gives an alkaline solution. For example, the aqueous solutions of both sodium carbonate (Na2CO3) and sodium acetate (CH3COONa) are alkaline.
Na2CO3 + 2H2O → 2NaOH + CO2 + H2O
CH3COONa + H2O → CH3COOH + NaOH
(iii) A salt derived from a strong acid and a weak base gives an acidic solution. For example, both aluminium chloride (AlCl3) and ammonium chloride (NH4Cl) make acidic aqueous solutions.
AlCl3 + 3H2O → Al(OH)3 + 3HCl
NH4Cl + H2O → NH4OH + HCl
(iv) Solutions of acidic salts are acidic to litmus, i.e., these solutions turn blue litmus paper red. For example, a solution of sodium hydrogensulphate (NaHSO4) turns blue litmus paper red.
Sodium hydrogencarbonate (NaHCO3) solution, however, is slightly alkaline.
