General Properties of Bases
Some of the characteristic properties of bases are:
- Bases are bitter to taste a bitter taste is characteristic of all bases.
- Bases may or may not be soluble in water Bases that can dissolve in water are called alkalis. Some examples of soluble bases or alkalis are sodium hydroxide, potassium hydroxide, and calcium hydroxide.
Like acids, bases can be strong or weak.
Strong bases are very corrosive and can burn the skin.
These bases should be handled carefully. Caustic soda or sodium hydroxide (NaOH) and caustic potash or potassium hydroxide (KOH) are strong and corrosive bases.
On the other hand, copper hydroxide [Cu(OH)2], zinc hydroxide [Zn(OH)2], and ammonium hydroxide (NH4OH) are weak bases.

- The solutions of bases in water give a soapy touch. When dissolved in water they produce hydroxide ions (OH–) in solution.
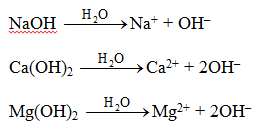
- They turn red litmus paper blue.
Take some soap solution in a test tube. Dip the tip of a red litmus paper into it. You will see that red litmus paper turns blue. This indicates that the soap solution contains a base. - They react with acids to produce salt and water.
NaOH + HCI → NaCl + H2O
2KOH + H2SO4 → CuSO4 + 2H2O
Cu(OH)2 + H2SO4 → CuSO4 + 2H2O
In these reactions, the acid and the base neutralize each other. Therefore, these reactions are called neutralization reactions.
Thus, a neutralization reaction may be defined as a reaction between an acid and a base, producing salt and water.
This neutralization reaction may be explained as follows. You know, all acids provide H+ ions and all bases provide OH– ions in aqueous solution. Let us see what happens when HCl and NaOH react together.
HCl + NaOH → NaCl + H2O
or H+ + Cl– + Na+ + OH– → Na+ + Cl– + H2O
or H+ + OH– → H2O
Thus, during neutralization of an acid with a base or vice versa H+ ions (from acid) and OH– ions (from base) combine to produce H2O molecules. - The oxides which produce acids in aqueous solutions are called acidic oxides which are usually the oxides of nonmetals. Acidic oxides react with bases to give salts and water.
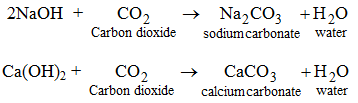
- When a base is heated with an ammonium salt, ammonia gas, another salt and water are produced. For example, when sodium hydroxide is heated with ammonium chloride, the products formed are sodium chloride, water and ammonia gas.
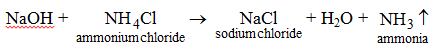
Ammonia gas is recognized by its pungent smell. - Bases react with certain salts to produce another salt and another base. For example, when NH4OH is added to a solution of Al2(SO4)3, (NH4)2SO4 and Al(OH)3 are produced.
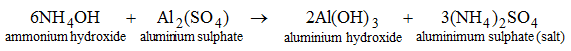
Bases need water to show basic properties
- A base needs to dissolve in water to show basic properties. Bases in dry condition or dissolved in organic solvents do not exhibit basic properties.
- A base needs water to produce hydroxide ion which is responsible for basic properties.
- Ammonia is a base. It consists of ammonia molecules.
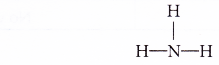
- Ammonia does not contain OH– ions. It needs water to help it to ionise to produce OH– ions.
- Experiment studies the need of water for ammonia to show its basic properties.
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution
Bases need water to show basic properties experiment

Aim: To investigate whether a base needs water to show its basic properties.
Problem statement: Does a base need water to show its basic properties?
Hypothesis: A base needs water to show its basic properties.
Variables:
(a) Manipulated variable : Types of solvents
(b) Responding variable : Change in colour of red litmus paper
(c) Controlled variable : Type of base
Operational definition: A red litmus paper is used to test if a solution is alkaline. Alkaline solutions turn red litmus paper blue.
Materials: Ammonium chloride, calcium hydroxide, calcium oxide, distilled water, trichloromethane and red litmus paper.
Apparatus: Test tubes, boiling tube, beakers, filter funnel, delivery tubes, Bunsen burner, U-tube, stopper, dropper and test tube rack.
Procedure:
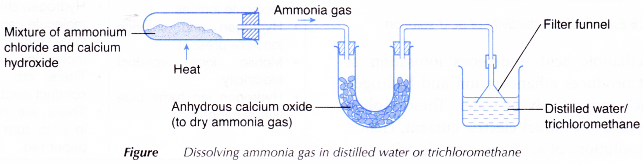
- A solution of ammonia in water and a solution of ammonia in trichloromethane are prepared using the apparatus set-up shown below.
- Two test tubes are labelled and placed in a test tube rack.
- About 1 cm3 of the solution of ammonia in water is placed in the first test tube (I).
- About 1 cm3 of the solution of ammonia in trichloromethane is placed in the second test tube (II).
- A piece of dry red litmus paper is dropped into each of the two test tubes.
- Any changes that occur are observed and recorded in a table.
Observations:
Discussion:
- Ammonia gas is liberated when a mixture of ammonium salt is heated with an alkali.

- Ammonia gas is a covalent compound composed of ammonia molecules.
- In water, the ammonia molecules react with water and ionise to produce ammonium ions and hydroxide ions.
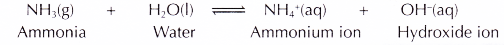
- The presence of mobile ions makes the aqueous solution of ammonia able to conduct electricity.
- The hydroxide ion turns red litmus paper blue.
- A solution of ammonia in trichloromethane is composed of only ammonia molecules. There are no ions present because the ammonia molecules cannot ionise in organic solvents. Without hydroxide ions, the solution does not show basic properties.
Conclusion:
The aqueous solution of ammonia shows basic properties. The hypothesis can be accepted.
