General Properties of Acids
Properties of Acids
Some of the characteristic properties of acids are:

- Acids are sour to taste and are corrosive in nature along with sour taste, acids also have the ability to corrode
metals such as iron and aluminium. For this reason, acids are generally stored in glassware.
Mineral acids such as nitric and sulphuric acids can burn human tissues upon contact and damage clothes, paper, etc. Thus, one should handle acids with care. - Acids are soluble in water Most acids dissolve in water either at room temperature or on heating to form a clear solution.
For example, vinegar is a 3-5% solution of acetic acid in water.
Depending on the amount of water, acids can be either dilute or concentrated.
If the amount of water is more in an acid, it is called dilute acid and if the amount of water is less, it is called concentrated acid. - Acids can be strong or weak. A strong add is highly corrosive and can cause severe burns. Nitric acid and sulphuric acids are a few examples of strong acids. Weak adds, on the other hand, are not as destructive as strong acids. Organic acids are generally weak acids.
- Acids show acidic properties only in the presence of water. This can be demonstrated by the following activity.
Dry hydrogen chloride gas does not produce H+ ions in the absence of moisture/water. It produces H+ ions only in the presence of moisture/water.
HCl + H2O → H3O+ + Cl– - Their aqueous solutions conduct electricity.
- They react with certain metals with the evolution of hydrogen gas.
Examples :
Metals like potassium, sodium, calcium, magnesium, aluminium, zinc and iron can react with the aqueous solution of an acid to evolve hydrogen gas.
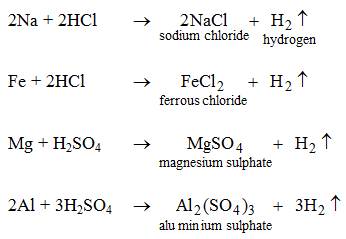
These reactions show certain metals can displace hydrogen from acids to form salts.
Nitric acid reacts only with magnesium and manganese. In both the reactions hydrogen gas is evolved.
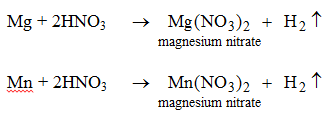
Nitric acid does not behave like this with any other metal. - They can react with bases to produce salts and water. [We will study these reactions when we deal with the bases.]
- They react with carbonates and hydrogencarbonates to form carbon dioxide and a salt.
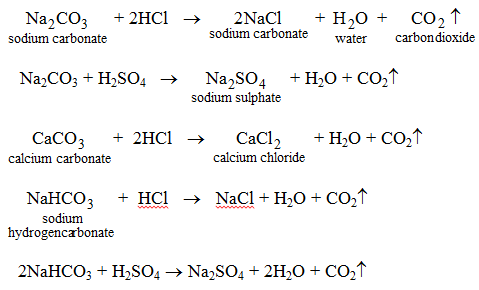
This activity may be used as a method of determining if a given substance is an acid or not.
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution
Acids need water to show acidic properties
- An acid only shows its acidic properties when it is dissolved in water. Water helps the acid molecules to ionise to produce hydrogen ion.
- It is the hydrogen ion that causes acidity.
- Experiment shows the need of water for an acid to show its acidity.
- Figure shows the types of particles present in ethanoic acid under different conditions.
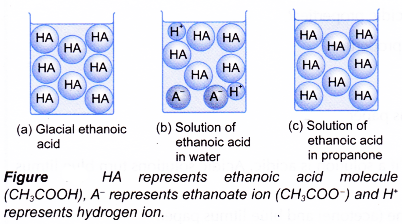
- Glacial ethanoic acid is pure ethanoic acid. It consists of only CH3COOH molecules. Therefore, it does not show acidity.
- If glacial ethanoic acid is dissolved in water, it ionises to form hydrogen ion. The hydrogen ion causes the acidity in aqueous ethanoic acid.
- (a) Ethanoic acid is a covalent compound. It is thus soluble in organic solvents such as propanone, trichloromethane, tetrachloromethane, 1,1,1 – trichloroethane and methylbenzene.
(b) However, no ionisation occurs. The ethanoic acid remains as unionised molecules. Without the presence of hydrogen ions, a solution of ethanoic acid in organic solvent cannot exhibit acidic properties. - The ionisation is shown by the electrical conductivity of the acid solution (Figure).
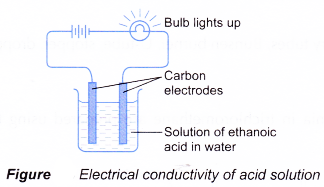
When ethanoic acid undergoes ionisation in water, it produces ethanoate ion and hydrogen ion which move freely in water. These mobile ions are able to conduct electric current. Hence, aqueous solutions of acids conduct electricity. - Hydrogen chloride gas is prepared in the laboratory by reacting sodium chloride with concentrated sulphuric acid.
NaCl(s) + H2SO4(aq) → NaHSO4(aq) + HCl(g) - The hydrogen chloride gas is then dissolved in water or methylbenzene using the set-up shown below.
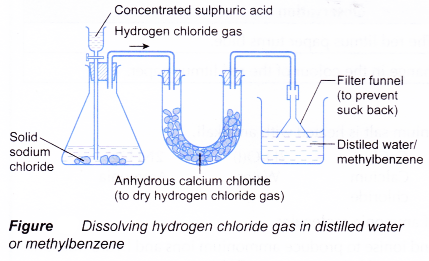
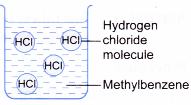
- Table shows a comparison of the properties of hydrogen chloride dissolved in water and in an organic solvent.
| Solution of hydrogen chloride in water | Solution of hydrogen chloride in methylbenzene |
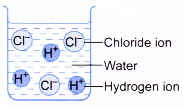
| 
|
Acids need water to show acidic properties experiment
Aim: To investigate whether water is needed for an acid to show its acidic properties.
Problem statement: Does an acid need water to show its acidic properties?
Hypothesis: Water is needed for an acid to show its acidic properties.
Variables:
(a) Manipulated variable : Types of solvents
(b) Responding variable : Change in colour of blue litmus paper
(c) Controlled variable : Type of acid
Operational definition: A blue litmus paper is used to test if a solution is acidic. Acidic solutions turn blue litmus paper red.
Materials: Glacial ethanoic acid, distilled water, propanone (acetone) and blue litmus paper.
Apparatus: 3 test tubes, dropper and test tube rack.
Procedure:
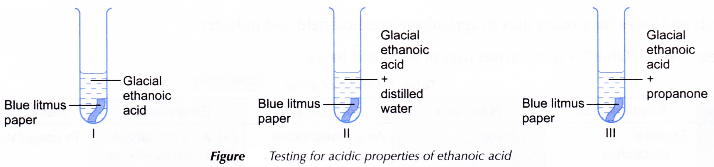
- Three test tubes are labelled and placed in a test tube rack.
- About 1 cm3 of glacial ethanoic acid is placed into each of the three test tubes.
- About 2 cm3 of distilled water is added to test tube II and the mixture is shaken.
- About 2 cm3 of propanone is added to test tube III and the mixture is shaken.
- A piece of dry blue litmus paper is dropped into each of the three test tubes.
- Any changes that occur are observed and recorded in a table.
Observations:
| Test tube | Observation |
| I | No visible change in the colour of the litmus paper, remains blue. |
| II | The blue litmus paper turns red. |
| III | No visible change in the colour of the litmus paper, remains blue. |
Discussion:
- Glacial ethanoic acid contains pure ethanoic acid. It consists entirely of ethanoic acid molecules. Without hydrogen ions, glacial ethanoic acid cannot show acidic properties.
- The presence of water helps the ethanoic acid molecule to ionise to produce hydrogen ion. The hydrogen ion turns blue litmus paper red.
- Propanone is an organic solvent. It does not allow the ethanoic acid molecule to ionise. The ethanoic acid remains as a molecule. Without the presence of hydrogen ions, the blue litmus paper cannot turn red.
- An acid needs water for it to ionise to produce hydrogen ions to exhibit acidic properties.
Conclusion:
Ethanoic acid shows acidic properties when it is dissolved in water. The hypothesis can be accepted.
