What is the Flame of a Candle made of
Flame
A flame is a region where combustion of fuel takes place. The colour of the flame depends on the temperature, amount of air available, and the nature of the substance burning. Hydrocarbons burn with a blue or yellow flame. Figure shows the change in flame colour of a Bunsen burner with increasing oxygen supply.

A yellow flame is also called a luminous flame, as it emits a lot of light. A luminous flame is generally observed when there is insufficient oxygen (i.e., incomplete combustion). Its temperature is lower than that of a blue flame and it leaves behind black soot and other residue.
A blue flame is also called a non-luminous flame as it emits very little light. A blue flame is generally observed when there is adequate amount of oxygen available (i.e., complete combustion). This type of flame leaves behind no residue.
However, a flame may not show the same colour uniformly. We sometimes observe different colours or zones in a flame. Let us understand this using the example of a candle flame.
Zones of a Candle Flame
A candle flame can be divided into three zones, depending on the amount of oxygen available.
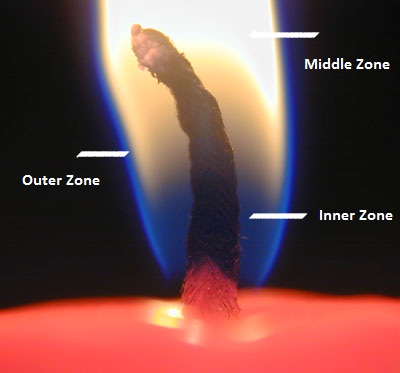
- The outer zone (blue) is the hottest part of the flame, in this zone, the wax vapours have enough oxygen to burn completely (producing carbon dioxide and water). This zone emits very little light.
- The middle zone (yellow) is less hot than the outer zone. Here, incomplete combustion of wax vapours (due to low oxygen) produces carbon particles (which glow, giving the zone its yellow colour) and carbon monoxide. This zone emits the most light.
- The inner zone (black) is the coolest part of the flame. In this zone, the wax vapours remain unburned as no oxygen is available. This zone is completely dark and emits no light.
