What are fats and oils?
Fats
- Fats and oils belong to a group of naturally occurring substances called lipids.
- Lipids are a group of organic compounds that make up the structure of cells found in plant and animal tissues. The lipids are water-insoluble compounds that can be extracted from cell components by using organic solvents such as ether and benzene.
- Fats and oils are chemically very similar, but differ in their physical states.
- Fats found in animals like goats and cows are solids at room temperature. Examples of animal fats are butter and tallow.
- Fats from plants are liquids. They are called oils. Palm oil, coconut oil, olive oil, soybean oil and corn oil are examples of vegetable oils.
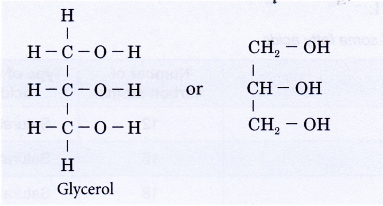
- Fats and oils are mixtures of different esters derived from a variety of long-chain carboxylic acids called fatty acids with the alcohol propane-1,2,3-triol (commonly known as glycerol).
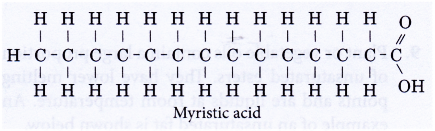
- Fatty acids are long straight-chain carboxylic acids containing between 12 to 18 carbon atoms per molecule. An example is myristic acid shown below.
- A molecule of glycerol may combine with one, two or three fatty acids to form a monoester, diester or triester. A molecule of water is eliminated when a fatty acid joins to the glycerol molecule and the resulting bond formed is called an ester link (-COO-).
- Most fats and oils are triesters (triglyceride) and have the general structure shown below.
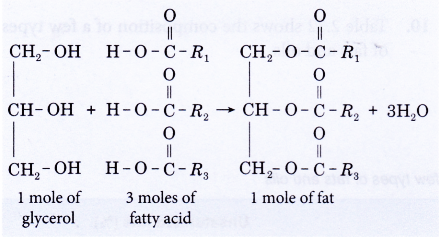
where the three alkyl groups R1, R2 and R3 may be the same or different.
Why does our body need oils and fats?
- Source of energy
Fats and oils serve as the most efficient means for living things to store energy. They are thus an important source of energy. Like carbohydrate, fat releases energy when it is oxidised to carbon dioxide and water. They supply us with more energy per gram than carbohydrates. - Source of nutrients
We need to include vitamins as part of a balanced diet to maintain good health. Vitamins A, D, E and K are insoluble in water but soluble in fats. We can meet our vitamin requirements by consuming foods that contain these fat-soluble vitamins. - Structural role
Fat plays important structural roles in the body. The protective membrane of each body cell is made from fat molecules. Even the nucleus of the cell is protected by layers of fat molecules. - Thermal insulation
The layer of fat under our skin is to protect us from the cold and to protect our internal organs from freezing. The layer of fat under the skin acts as a thermal insulator. - Protection
Fat surrounds and protects vital internal organs in our body. For example, kidney is protected by a layer of fat.
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
What are some examples of saturated and unsaturated fats?
Saturated and unsaturated fats
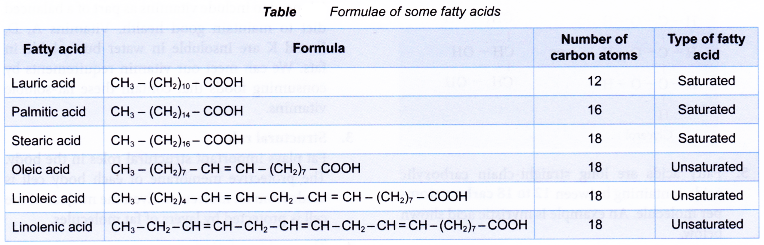
- The physical and chemical properties of a fat or oil molecule are greatly affected by the parent fatty acids. The fatty acids can be different in two main ways.
- Firstly, the length of the carbon chain can differ, ranging from 12 to 18 carbon atoms. For example, lauric acid (12 carbon atoms) and stearic acid (18 carbon atoms) have different numbers of carbon atoms.
- Secondly, the fatty acid may be saturated or unsaturated. A saturated fatty acid has all carbon atoms joined together by carbon-carbon single bonds. Palmitic acid is a saturated fatty acid.
- In an unsaturated fatty acid, the carbon chain has one or more carbon-carbon double bonds. If the fatty acid molecule has one carbon-carbon double bond, it is described as monounsaturated. Oleic acid is a monounsaturated fatty acid containing one carbon-carbon double bond.
- If there is more than one carbon-carbon double bond, the fatty acid is described as polyunsaturated. Linoleic acid has two carbon- carbon double bonds, whereas linolenic acid has three carbon-carbon double bonds.
The formulae of some fatty acids are given in Table.
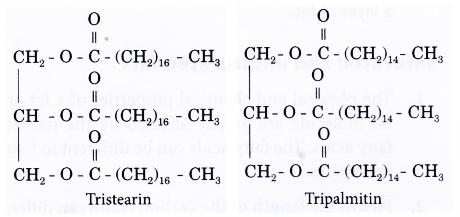
- Fats which contain esters of glycerol and saturated fatty acids are classified as saturated fats. Animal fats containing a large proportion of saturated esters tend to have high melting points and are solids at room temperature. Two saturated fats are given below.
- Fats which contain esters of glycerol and unsaturated fatty acids acids are classified as unsaturated fats.
- Plant or vegetable oils contain a large proportion of unsaturated esters. They have lower melting points and are liquids at room temperature. An example of an unsaturated fat is shown below.
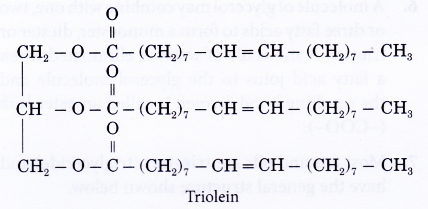
Table shows the composition of a few types of fats and oils.
Source | Saturated fats (%) | Unsaturated fats (%) | |||||
| Myristic acid | Palmitic acid | Stearic acid | Others | Oleic acid | Linoleic acid | Others | |
| Butter | 10 | 25 | 10 | 21 | 25 | 5 | 4 |
| Animal fats | 4 | 29 | 19 | – | 44 | 3 | 1 |
| Groundnut oil | – | 7 | 5 | – | 60 | 20 | 8 |
| Olive oil | 1 | 5 | 5 | – | 80 | 7 | 2 |
| Palm oil | – | 44 | 5 | – | 40 | 10 | 1 |
Converting unsaturated fats to saturated fats
- Unsaturated fats can be converted into saturated fats by a process called catalytic hydrogenation. Each carbon-carbon double bond absorbs one mole of hydrogen.
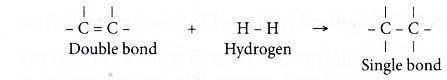
- The hydrogenation process is carried out by bubbling hydrogen gas through hot liquid oil in the presence of fine particles of nickel catalyst. A temperature of about 200°C and a pressure of about 4 atm are used.
- The relative molecular mass of the oil molecule increases, as more and more of the double bonds are hydrogenated. Intermolecular forces become stronger and more energy is needed to overcome them. The boiling point of the oil increases and the physical state changes from liquid to solid.
- Margarine is a soft solid with low melting point at room temperature. It is made by hydrogenating some of the carbon-carbon double bonds in polyunsaturated vegetable oil such as palm oil and sunflower oil.
How do saturated and unsaturated fats affect the body?
Effect of fats on health
- Medical research has found that a diet high in animal fats, particularly saturated fats, is considered unhealthy.
(a) Fatty foods are very high in energy. One gram of fat releases about 9 kilocalories of energy. Hence, high consumption of food high in fats and oils is likely to result in obesity.
(b) An obese person is prone to suffer from heart disease and other diseases such as diabetes. - Medical researchers have found that animal fats pose a greater risk for coronary heart disease and strokes than vegetable oils. Saturated fats contain cholesterol.
(a) Consuming food high in saturated fats will raise the level of cholesterol in the bloodstream. It is the cholesterol which causes fatty deposits or plaque on the walls of veins or arteries. As the plaque builds up on the walls of the blood vessels, blood circulation is restricted and this will raise blood pressure.
(b) Hardening of the arteries occurs if the deposits take place in the heart arteries. A condition called arteriosclerosis occurs, preventing adequate supply of blood from reaching the heart and can cause heart attack.
(c) High-density lipoproteins (HDL) is called the ‘good’ cholesterol because it can reduce the risk of heart disease. High levels of low-density lipoproteins (LDL), the ‘bad’ cholesterol, increase the risk of heart disease. - Vegetable oils do not contain cholesterol because only animals make cholesterol. These unsaturated fats do not have the damaging effects of saturated fats to cause cardiovascular problems. This does not mean that we can consume unsaturated fats in large quantities. Too much fat, whether saturated or unsaturated, will make us overweight. A balanced healthy diet will keep the vital processes in our bodies to function efficiently to maintain a healthy body.
