How esters are formed?
What is an ester in chemistry?
Esters
- Esters are non-hydrocarbon organic compounds containing carbon, hydrogen and oxygen.
- The smallest member of the ester family is shown in Photograph.
- In fact, an ester is the product of an esterification reaction between a carboxylic acid and an alcohol. A molecule of water is eliminated when a carboxylic acid combines with an alcohol to form an ester.
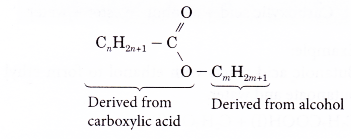
- The general formula of the esters is CnH2n+1COOCmH2m+1 with values of n = 0, 1, 2, 3,…, and m = 1, 2, 3,… The values of n and m indicate the numbers of carbon atoms in the ester molecule.
- The general formula contains the group –COO. This is known as the carboxylate group. This is the functional group of the esters and has the structure shown below.

How do you name an ester?
Naming esters
- Structure of an ester may be regarded as consisting of two parts, one deriving from an alcohol and the other from a carboxylic acid.
- Thus, the name of an ester consists of two parts. The name of the alcohol part of the ester is given first, and is followed by a separate word giving the name of the acid part of the ester.
- The name of the alcohol part of the ester is the name of the alkyl group (–CmH2m+1) in the alcohol.
- The name of the acid part of the ester is the name of the carboxylate anion (CnH2n+1–) derived from the acid. The -oic acid in the name of the parent acid is replaced by -oate.
- In general, names of esters are of the form ‘alkyl carboxylate’. The following example shows how to name an ester.
Naming ester example: Name the ester below.
CH3COOCH2CH2CH3
Solution:
Step 1: Identify and name the alcohol part of the ester.
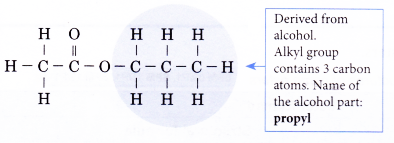
Step 2: Identify and name the acid part of the ester.
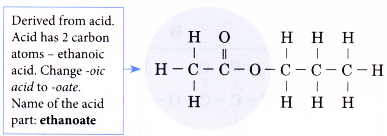
Step 3: Combine the two parts to obtain the name of the ester.
Name of the ester: propyl ethanoate
Remember: First, the alcohol part is named and then followed by the acid part. The name of an ester consists of two separate words.
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Which acid is used for coagulating rubber from latex?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
What is an esterification reaction?
Formation of esters
- Esters are produced by an esterification reaction. An esterification reaction involves a molecule of carboxylic acid with a molecule of alcohol in the presence of a strong acid catalyst such as concentrated sulphuric acid, to form an ester and water.
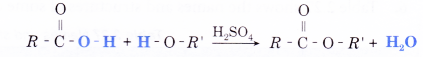
- For example, a molecule of methanoic acid combines with a molecule of methanol to form a molecule of methyl methanoate and a-molecule of water.
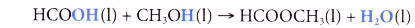
- On the other hand, water is also produced in a neutralisation reaction. However, this water is formed from ions; a reaction between a hydrogen ion and a hydroxide ion.
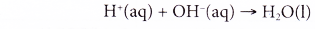
- For example, sodium hydroxide solution is neutralised by hydrochloric acid to produce sodium chloride (a salt) and water.
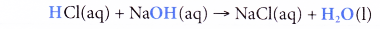
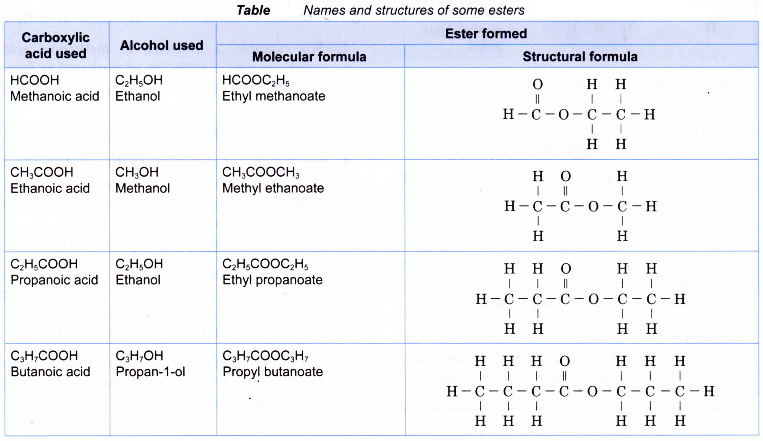
- The following example shows how to predict the esters formed from the esterification between a carboxylic acid and an alcohol.
Table shows the names and structures of some esters.
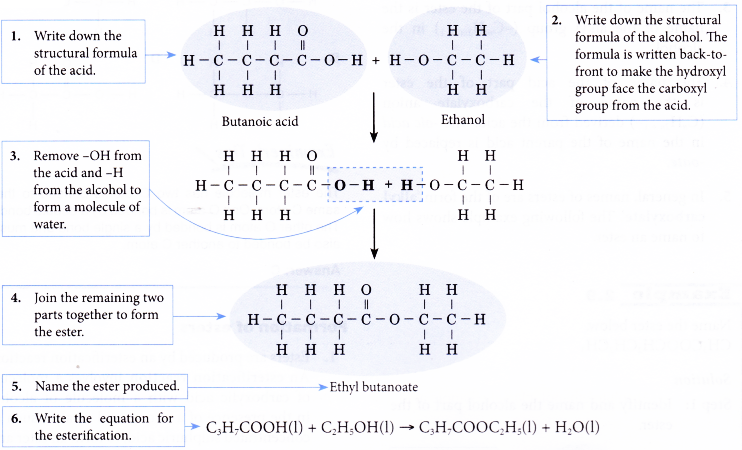
Formation of ester example: Predict the ester produced from an esterification reaction between butanoic acid and ethanol and write an equation for the esterification reaction.
Solution:
How do you make ethyl Ethanoate? Experiment
Aim: To prepare a sample of ethyl ethanoate in the laboratory.
Materials: Absolute ethanol, glacial ethanoic acid, concentrated sulphuric acid, oil, water.
Apparatus: Beakers, distillation flask, tap funnel, conical flask, measuring cylinder, thermometer, Liebig condenser, Bunsen burner, tripod stand, retort stand and clamp, stopper with two holes, wooden block.
Procedure:
- About 50 cm3 of absolute ethanol is measured into a beaker. About 50 cm3 of glacial ethanoic acid is added and mixed well. This mixture is poured into a distillation flask.
- About 25 cm3 of absolute ethanol is measured into a beaker. About 25 cm3 of concentrated sulphuric acid is slowly added with shaking to the absolute ethanol. The mixture is carefully poured into a tap funnel.
- The apparatus as shown in Figure is set up.
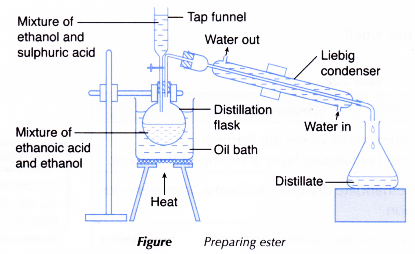
- The oil bath is heated to about 140°C and is maintained at this temperature throughout.
- The mixture from the tap funnel is dripped into the distillation flask at the same rate as the distillate collects in the conical flask.
- The distillate collected in the conical flask is observed.
Observations:
A colourless liquid with fruity smell is obtained.
Discussion:
- Ethanoic acid reacts with ethanol to form ethyl ethanoate and water.

- Concentrated sulphuric acid is used as a catalyst and to absorb water to push the reaction to produce more ester.
Conclusion:
An ester is produced from an esterification reaction between a carboxylic acid and an alcohol.
Physical properties of esters
- The most noticeable characteristic of the esters is their smell. The simple esters are neutral compounds with a sweet pleasant smell (fruity smell).
- These esters tend to be colourless liquids with boiling points much lower than those of carboxylic acids of similar molecular masses; making them a group of volatile compounds.
- They are slightly soluble in water but readily dissolve in organic solvents.
Aim: To investigate the physical properties of ethyl ethanoate.
Materials: Ethyl ethanoate, distilled water, acetone, methylated spirits.
Apparatus: Test tube, dropper, glass rod, sample bottles.
Procedure:
- About 2 cm3 of ethyl ethanoate is poured into a test tube. The smell of the ester is noted.
- About 5 cm3 of distilled water is added to the ester and the mixture is shaken well. The solubility of the ester in water is noted.
- Steps 1 to 2 are repeated using acetone and methylated spirits consecutively to replace distilled water.
Observations:
- Ethyl ethanoate has a fruity smell.
- Solubility:
| Solvent | Observation |
| Water |
|
| Acetone |
|
| Methylated spirits |
|
Conclusion:
Ethyl ethanoate is a colourless liquid with a fruity smell. It is soluble in organic solvent but cannot dissolve in water.
Do esters occur naturally?
Natural sources of esters
- Most simple esters are found in fruits and flowers. These volatile esters are responsible for the fruity smells and pleasant fragrances associated with most fruits and flowers.
Example:
Banana owes its smell to n-pentyl ethanoate. Pineapple gets its flavour from ethyl butanoate. - The higher and more complex esters have higher boiling points, and thus are less volatile. They do not produce the familiar pleasant smells of the simple esters.
- Animal fats such as milk fat are solid esters. Vegetable oils, for example palm oil, are liquid esters. Both fats and oils are esters of glycerol and fatty acids.
- Waxes are solid esters derived from long- chain fatty acids and long-chain alcohols. Beeswax is made from the ester with formula C15H31COOC30H61.
What is the use of esters?
Uses of esters in everyday life
- Esters have many uses in both the living world and industries.
- Natural fats are triesters of glycerol which serve as a storage reserve of energy in living things.
- Esters called parabens are used as food and drug preservatives because they can prevent the growth of microorganisms such as molds and yeast.
- The leaves and stems of some plants are coated with wax, which helps to prevent both dehydration and attack by microorganisms. The fur of animals and feathers of birds are also coated with wax to keep them dry.
- Esters with low molecular mass are volatile liquids and have sweet smells. Their pleasant aromas make them suitable for the preparation of cosmetics and perfumes. These esters are used as food additives to improve the flavour and smell of processed foods. They are listed as artificial flavours’ on labels of processed foods, snacks and confectionery. Table shows some artificial fruit flavourings.
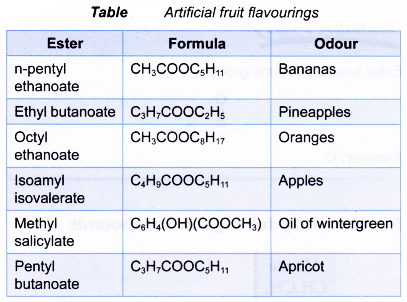
- The small esters like ethyl ethanoate are excellent solvents for organic compounds. Sunburn lotions, nail polish removers, plasticisers and glues use esters as solvents.
- For example, polystyrene cement is a mixture of polystyrene dissolved in ethyl ethanoate. When the ester evaporates, a solid plastic is left behind to bind together the surfaces being joined.
- Large esters are used in the production of soaps and detergents. Fats and oils are hydrolysed to produce soaps.
- Esters are also used in the production of polyester. The long-chain polyester molecules are unreactive and have great tensile strength. Hence, they are used to make polyester threads in the production of synthetic fabrics.
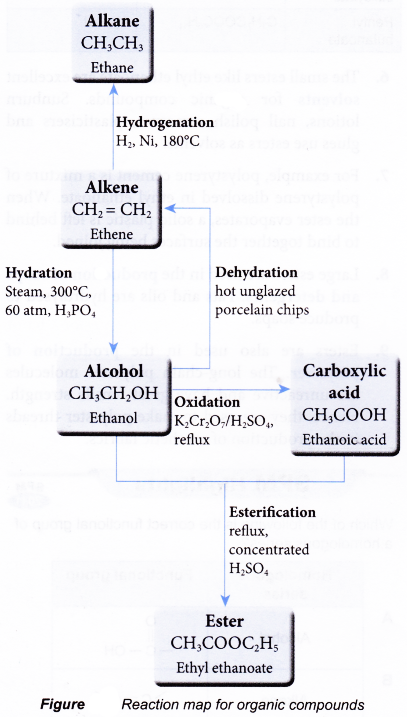
Reaction map for organic compounds