What is the enthalpy (heat) of neutralization?
- Neutralisation is the reaction between an acid and a base to form a salt and water.
- Some examples of neutralisation reaction are as follows.
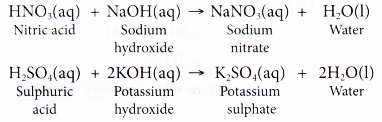
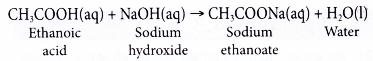
- During neutralisation reaction, hydrogen ions from acid react with hydroxide ions from alkali to form water.
H+(aq) + OH–(aq) → H2O(aq) - Since water is formed during neutralisation, heat of neutralisation is defined based on the formation of water.
- The heat of neutralisation is the heat produced when one mole of water is formed from the reaction between an acid and an alkali.
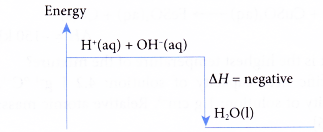
- Neutralisation is an exothermic reaction. Neutralisation always produces heat. Therefore, heat of neutralisation, AH is always negative.
- The energy level diagram for a neutralisation reaction is as shown below.
People also ask
- How can energy be changed in a chemical reaction?
- How does the energy level diagram show this reaction is exothermic?
- What is enthalpy of reaction?
- What is heat of precipitation?
- What is heat of displacement?
- What is the heat of combustion?
- Why is energy released when a bond is formed?
- Applications of exothermic and endothermic reactions in everyday life
- What is meant by the calorific value of a fuel?
Determine heat of neutralization of strong acid and strong base
Determining heat of neutralisation:
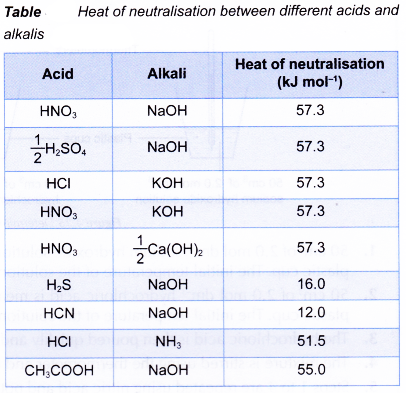
The heats of neutralisation between strong acids and strong alkalis are always the same.
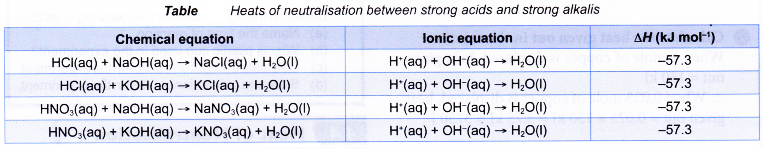
- The energy level diagram for neutralisation between a strong acid and a strong alkali is as shown below.
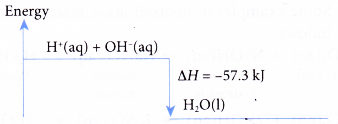
- The value of the heat of neutralisation depends on:
(a) The basicity of the acid
(b) The strength of the acid
(c) The strength of the alkali - Basicity of the acid
(a) Complete neutralisation of a strong diprotic acid with an alkali produces double amount of heat as compared to a strong monoprotic acid.
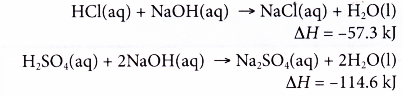
(b) This is because a diprotic acid like sulphuric acid produces two moles of hydrogen ions when it dissociates in water.
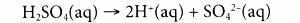
(c) Two moles of hydrogen ions produce two moles of water when reacted with hydroxide ions from an alkali.
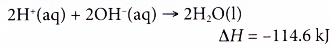
(d) Sulphuric acid is also a strong acid and the heat of neutralisation is equal to -57.3 kJ mol-1.
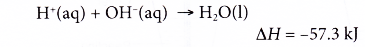
- Strength of the acid
(a) The heat given out when a strong acid reacts with a strong alkali is higher than the heat given out when a weak acid reacts with a strong alkali.
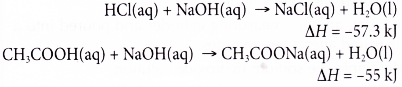
(b) Ethanoic acid is a weak acid that dissociates partially in water. Most of the ethanoic acid still exists as molecules when it dissolves in water.
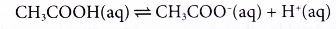
(c) Some of the heat given out during the neutralisation is used to dissociate the acid completely in water, thus the heat given out is always less than 57.3 kJ. - Strength of the alkali
(a) The heat given out when a strong acid reacts with a strong alkali is higher than the heat given out when a strong acid reacts with a weak alkali.
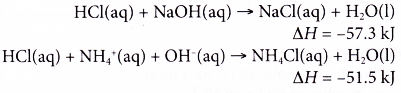
(b) Ammonia solution is a weak alkali which dissociates partially in water. Most of the ammonia still exists as molecules.
(c) Some of the heat given out during the neutralisation is used to dissociate the alkali completely in water, thus the heat given out is always less than 57.3 kJ. - The heat of neutralisation between a weak acid and a weak alkali is the least. This is because more energy is needed to dissociate both the weak acid and the weak alkali completely to produce hydrogen ions and hydroxide ions which then react together to form one mole of water.
Heat of Neutralisation Experiment
Aim: To determine the heat of neutralisation.
Materials: 2.0 mol dm-3 hydrochloric acid, 2.0 mol dm-3 sodium hydroxide solution, 2.0 mol dm-3 nitric acid, 2.0 mol dm-3 potassium hydroxide solution.
Apparatus: 50 cm3 measuring cylinders, thermometer, plastic cups with covers.
Safety measure:
Handle the chemicals with caution.
Wear eye protection.
Caution: Stir the mixture throughout the experiment.
Procedure:
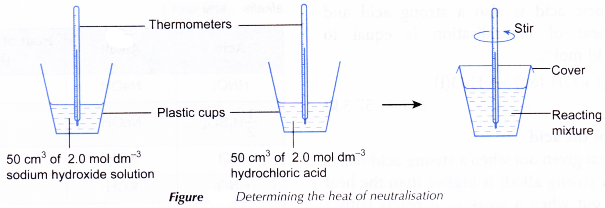
- 50 cm3 of 2.0 mol dm-3 sodium hydroxide acid is measured using another measuring cylinder and poured into a plastic cup. The initial temperature of the solution is measured after a few minutes.
- 50 cm3 of 2.0 mol dm-3 hydrochloric acid is measured using another measuring cylinder and poured into a plastic cup. The initial temperature of the solution is measured after a few minutes.
- The hydrochloric acid is then poured quickly and carefully into the sodium hydroxide solution.
- The mixture is stirred using the thermometer and the highest temperature reached is recorded.
- Steps 1 to 4 are repeated using nitric acid and potassium hydroxide solution to replace hydrochloric acid and sodium hydroxide solution with other factors remain unchanged.
Results:
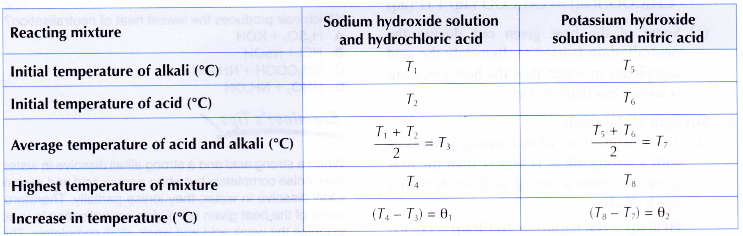
Interpreting data:
1. Reaction between sodium hydroxide solution and hydrochloric acid


2. Reaction between potassium hydroxide solution and nitric acid
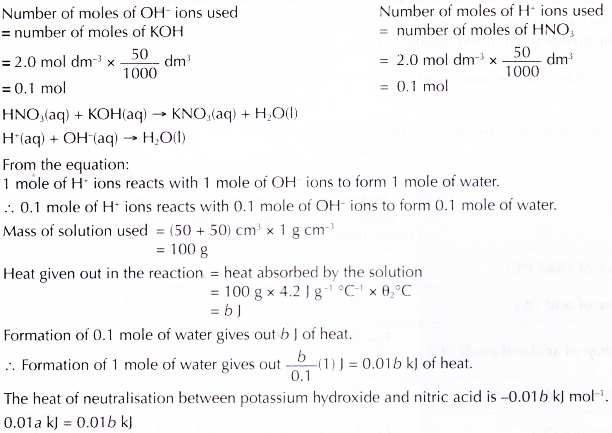
Discussion:
- It is found that the heat of neutralisation between sodium hydroxide and hydrochloric acid and the heat of neutralisation between potassium hydroxide and nitric acid are the same. This is because both the reactions are between a strong monoprotic acid and a strong alkali.
H+(aq) + OH–(aq) → H2O(l) ΔH = -57.3 kJ - It is found that the value of heat of neutralisation obtained in the experiment is less than the theoretical value, ΔH = -57.3 kJ mol-1. This is because some heat is lost to the surroundings.
- It is necessary to mix the acid and the alkali quickly to reduce heat loss to the surroundings.
- A plastic cup is used to reduce heat loss to the surroundings.
Conclusion:
The heat of neutralisation between a strong monoprotic acid and a strong alkali is -57.3 kJ mol-1.
Determine heat of neutralization of between acid and base experiment
Aim: To determine and compare the heats of neutralisation between acids and alkalis of different strength.
Materials: 2.0 mol dm-3 hydrochloric acid, 2.0 mol dm-3 sodium hydroxide solution, 2.0 mol dm-3 ethanoic acid, 2.0 mol dm-3 ammonia solution.
Apparatus: 50 cm3 measuring cylinders, thermometer, plastic cups with covers.
Procedure:
- 50 cm3 of 2.0 mol dm-3 sodium hydroxide solution is measured using a measuring cylinder and poured into a plastic cup. The initial temperature of the solution is measured after a few minutes.
- 50 cm3 of 2.0 mol dm-3 hydrochloric acid is measured using another measuring cylinder and poured into a plastic cup. The initial temperature of the solution is measured after a few minutes.
- The hydrochloric acid is then poured quickly and carefully into the sodium hydroxide solution.
- The mixture is stirred using a thermometer and the highest temperature reached is recorded.
- Steps 1 to 4 are repeated using
- Sodium hydroxide solution and ethanoic acid
- Ammonia solution and hydrochloric acid
- Ammonia solution and ethanoic acid
Results:
1. The results of the experiment are shown in the table below.
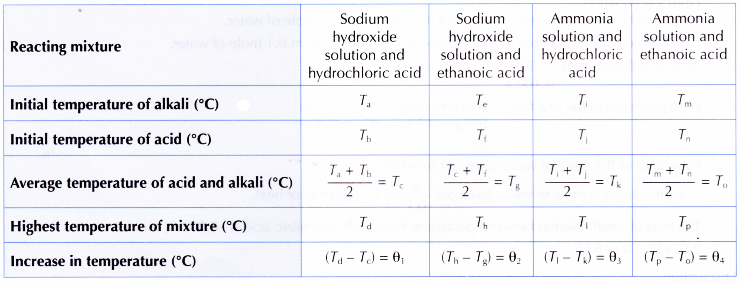
2. The temperatures of all the resulting mixtures increase.
3. The increase in the temperature of the reacting mixture is in the order of θ1 > θ2 > θ3 > θ4.
Discussion:
- The heat of neutralisation for the reaction between a strong acid and a strong alkali is the highest, whereas the heat of neutralisation for the reaction between a weak acid and a weak alkali is the lowest.
- The heat of neutralisation for the reactions between acids and alkalis decreases in the order:
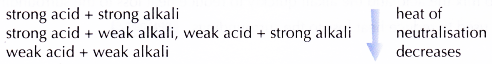
- Ethanoic acid is a weak acid and ammonia solution is a weak alkali, they both dissociate partially when dissolved in water. Most of the ethanoic acid and ammonia solution still exist as molecules.
- Some of the heat given out during the neutralisation reaction is used to dissociate the weak acid or the weak alkali completely in water.
- For the reaction between ethanoic acid and ammonia solution, the heat of neutralisation is the lowest. This is because much more energy is needed to dissociate both the weak acid and the weak alkali completely to produce hydrogen ions and hydroxide ions which then react together to form one mole of water.
- The equations for the neutralisation reactions are as follows.
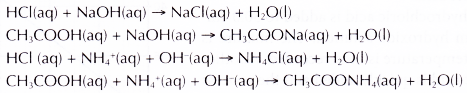
- It is necessary to mix the acid and the alkali quickly to reduce heat loss to the surroundings.
- A plastic cup is used in this experiment to reduce heat loss to the surroundings.
Conclusion:
The heat of neutralisation is the highest for the reaction between a strong acid and a strong alkali, and is the lowest for the reaction between a weak acid and a weak alkali.
How to calculate heat of neutralization problems with solutions
1. In an experiment to determine the heat of neutralisation, 50 cm3 of 1.0 mol dm-3 sulphuric acid at 28.5°C is added to 50 cm3 of 2.0 mol dm-3 potassium hydroxide solution which is also at 28.5°C in a plastic cup with a cover. The mixture is then stirred and the highest temperature reached is 41.5°C. Calculate the heat of neutralisation.
[Specific heat capacity of solution: 4.2 J g-1 °C-1; density of solution: 1 g cm-3]
Solution:
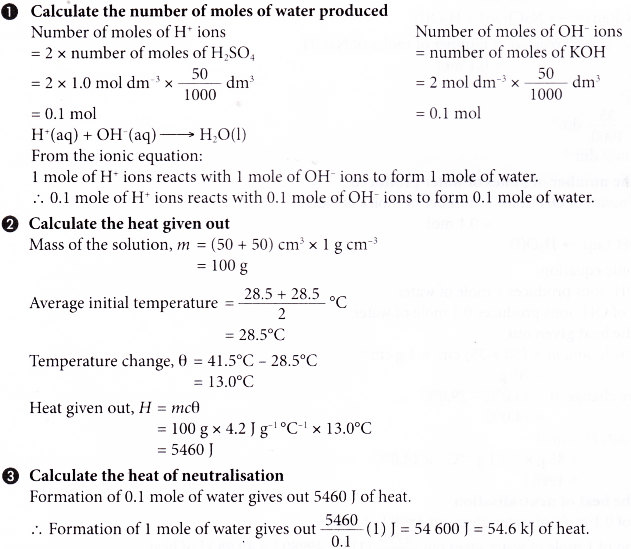
The heat of neutralisation between sulphuric acid and potassium hydroxide solution is -54.6 kJ mol-1.
2. A student carried out an experiment to investigate the change in temperature during a titration between sodium hydroxide solution and hydrochloric acid.
5.0 cm3 of m mol dm-3 hydrochloric acid is added to 50.0 cm3 of 2.0 mol dm-3 sodium hydroxide solution. The mixture is stirred and the highest temperature is then recorded. Another 5.0 cm3 of hydrochloric acid is quickly added and the process is repeated until a total of 50.0 cm3 of the acid is added. The results of the experiment are shown in Figure.
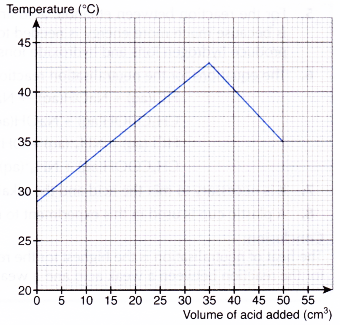
(a) (i) What is the initial temperature of the sodium hydroxide solution?
(ii) What is the highest temperature of the mixture?
(b) What is the volume of hydrochloric acid at the end point?
(c) What is the value of m?
(d) What is the heat of neutralisation?
[Specific heat capacity of solution: 4.2 J g-1 °C-1; density of solution: 1 g cm-3]
Solution:

The heat of neutralisation between hydrochloric acid and sodium hydroxide solution is -49.98 kJ mol-1.
3. The thermochemical equation for the reaction between nitric acid and sodium hydroxide solution is as shown below.
HNO3,(aq) + NaOH(aq) → NaNO3(aq) + H2O(l) ΔH = -57.3 kJ
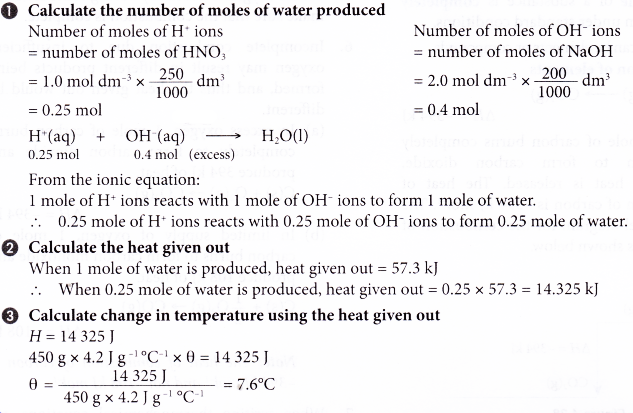
When 250 cm3 of 1.0 mol dm-3 nitric acid is added to 200 cm3 of 2.0 mol dm-3 sodium hydroxide solution, what is the change in temperature?
[Specific heat capacity of solution: 4.2 J g-1 °C-1; density of solution: 1 g cm-3]
Solution: