What is the heat of combustion?
What is the definition of enthalpy of combustion?
Heat of Combustion:
- A fuel is a chemical substance that burns in oxygen to produce heat energy.
- Combustion is a chemical reaction between a fuel and oxygen to release heat. Combustion is always an exothermic reaction.
- The heat of combustion is the heat produced when one mole of a substance is completely burnt in oxygen under standard conditions.
- The substances can be elements or compounds.
(a) Combustion of elements
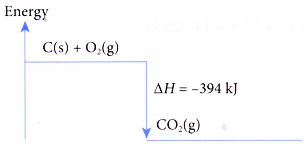
C(s) + O2(g) → CO2(g) ΔH = -394 kJ
When 1 mole of carbon burns completely in oxygen to form carbon dioxide, 394 kJ of heat is released. The heat of combustion of carbon is -394 kJ mol-1. The energy level diagram for the combustion of carbon is as shown below.
(b) Combustion of compounds
CH4(g) + O2(g) → CO2(g) + 2H2O(l)
When 1 mole of methane burns completely in oxygen to form carbon dioxide and water, 890 kJ of heat is released. The heat of combustion of methane is -890 kJ mol-1. The energy level diagram for the combustion of methane is as shown in Figure.

- Excess oxygen is necessary during combustion to make sure that the combustion is complete.
- Incomplete combustion due to insufficient oxygen may result in different products being formed, and thus the heat given out would be different.
(a) In excess oxygen, 1 mole of carbon burns completely to form carbon dioxide and produce 394 kJ of heat.
C(s) + O2(g) → CO2(g) ΔH = -394 kJ
(b) In limited supply of oxygen, 1 mole of carbon burns to form carbon monoxide and produce 108 kJ of heat.
C(s) + ½ O2(g) → CO2(g) ΔH = -108 kJ
Note: The heat of combustion of carbon is -394 kJ mol-1 and not -108 kJ mol-1. - When writing thermochemical equations, we can use fractions to represent the number of moles of oxygen required so that the number of moles of the substance is always 1 mole.
- Different fuels have different values of heat of combustion.
- The value of heat of combustion is an important factor for us to decide what fuel to use for a certain purpose.
People also ask
- How can energy be changed in a chemical reaction?
- How does the energy level diagram show this reaction is exothermic?
- What is enthalpy of reaction?
- What is heat of precipitation?
- What is heat of displacement?
- What is the enthalpy of neutralization?
- Why is energy released when a bond is formed?
- Applications of exothermic and endothermic reactions in everyday life
- What is meant by the calorific value of a fuel?
Determination of heat combustion by bomb calorimeter
Determining the heat of combustion:
- The heat of combustion of a fuel can be determined accurately by using a bomb calorimeter.
- An insulated container is filled with a known quantity of water. A small sample of known mass in the steel bomb is ignited electrically and burnt completely. The heat given out in the reaction is determined from the temperature rise in the water that surrounds the bomb.
- The bomb calorimeter is specifically designed to minimise heat loss to the surroundings.
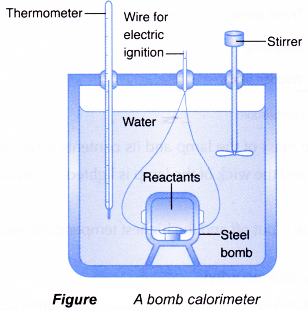
In the laboratory, the heat of combustion of a fuel can be determined as follows.
- A liquid fuel is burnt in excess oxygen.
- The mass and the number of moles of the fuel are determined.
- The heat produced is used to heat a known mass of water.
- The increase in the temperature of the water is measured.
- The heat absorbed by the water is calculated using the formula H = mcθ.
- The heat given out by the fuel is calculated. We assume that all the heat given out during the combustion of the fuel is absorbed by the water.
Heat given out during combustion of the fuel = heat absorbed by the water - The heat of combustion (heat given out by 1 mole of the fuel) is calculated.
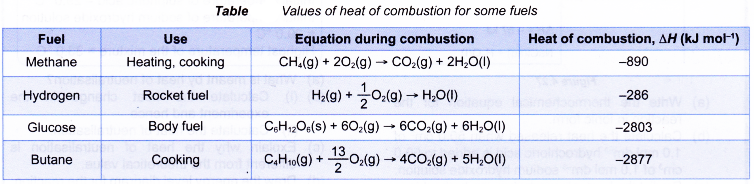
Heat of combustion of various alcohols
One example of liquid fuel is alcohol. Different members of the alcohol family have different heat of combustion. The table below shows the heat of combustion of some alcohols.
| Alcohol | Molecular formula | Number of carbon atoms per molecule | Number of hydrogen atoms per molecule | Relative molecular mass | Heat of combustion (kJ mol-1) |
| Methanol | CH3OH | 1 | 4 | 32 | -728 |
| Ethanol | C2H5OH | 2 | 6 | 46 | -1376 |
| Propan-1-ol | C3H7OH | 3 | 8 | 60 | -2016 |
| Butan-1-ol | C4H9OH | 4 | 10 | 74 | -2678 |
| Pentan-1-ol | C5H11OH | 5 | 12 | 88 | -3332 |
| Hexan-1-ol | C6H13OH | 6 | 14 | 102 | -3981 |
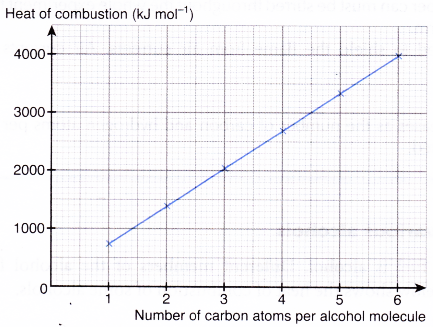
Table shows that the heat of combustion of alcohol increases as the
- number of carbon atoms per molecule increases
- number of hydrogen atoms per molecule increases
- relative molecular mass increases
During the combustion of alcohol:
- Carbon atom is burnt to form carbon dioxide.
[C] + O2(g) → CO2(g) + heat - Hydrogen atom is burnt to form water.
[H] + ½ O2(g) → H2O(g) + heat - Both the reactions are exothermic. Therefore, more heat is produced when more carbon and hydrogen atoms are burnt.
- Relative molecular mass is proportional to the number of carbon and hydrogen atoms per molecule. Thus, the heat of combustion of alcohol increases as the relative molecular mass increases.
The difference in the heat of combustion of successive members is almost the same, that is, about 650 kJ.
| Pair of alcohols | Difference in the heat of combustion (kJ) |
| Methanol and ethanol | 1376 – 728 = 648 |
| Ethanol and propan-1-ol | 2016 – 1376 = 640 |
| Propan-1-ol and butan-1-ol | 2678 – 2016 = 662 |
| Butan-1-ol and pentan-1-ol | 3332 – 2678 = 654 |
| Pentan-1-ol and hexan-1-ol | 3981 – 3332 = 649 |
(a) Successive members of the homologous series of alcohols differ from each other by a -CH2 group.
(b) The constant increase in the heat of combustion of the successive members of alcohol is due to the extra heat given out by the extra one carbon atom and two hydrogen atoms in the -CH2 group.
When the heat of combustion is plotted against the number of carbon atoms per alcohol molecule, the graph in Figure is obtained.
Heat of combustion of alcohols experiment
Aim: To investigate whether an alcohol with a higher number of carbon atoms per molecule has a higher heat of combustion.
Problem statement: Does an alcohol with a higher number of carbon atoms per molecule have a higher heat of combustion?
Hypothesis: The higher the number of carbon atoms per alcohol molecule, the higher is the heat of combustion.
Variables:
(a) Manipulated variable : Different types of alcohols/Number of carbon atoms per molecule of alcohol
(b) Responding variable : Heat of combustion
(c) Controlled variables : Volume of water, copper can, thermometer
Materials: Methanol, ethanol, propan-1-ol, butan-1-ol, water.
Apparatus: Copper can, tripod stand, thermometer (0 – 100°C), 100 cm3 measuring cylinder, spirit lamps, electronic balance, pipe-clay triangle, windshield, wooden block.
Procedure:
- 200 cm3 of water is measured using a measuring cylinder and poured into a copper can.
- The copper can is placed on a tripod stand.
- The initial temperature of the water is measured and recorded.
- A windshield is placed as shown in Figure.
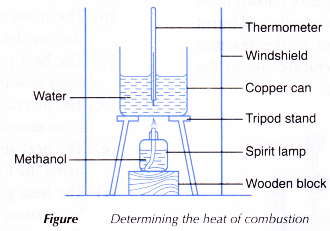
- About 50 cm3 of methanol is poured into a spirit lamp and the mass of the lamp and its contents is recorded.
- The lamp is put under the copper can as shown in Figure and the wick of the lamp is lighted immediately.
- The water is stirred throughout the experiment.
- When the temperature of the water increases by 30°C, the flame is put off and the highest temperature reached by the water is recorded.
- The mass of the lamp and its contents is weighed immediately and recorded.
- Steps 1 to 9 are repeated using ethanol, propan-1-ol and butan-1-ol to replace methanol with other factors remain unchanged.
Results:
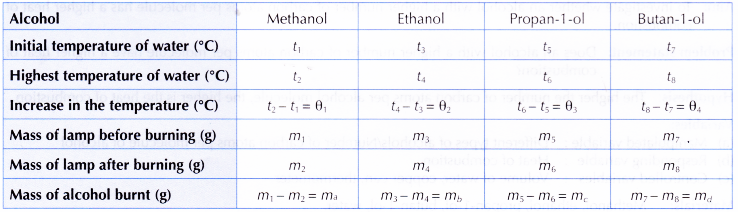
Interpreting data:
1. Heat of combustion of methanol
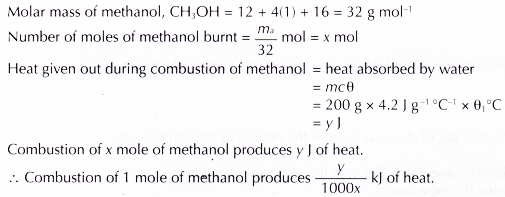
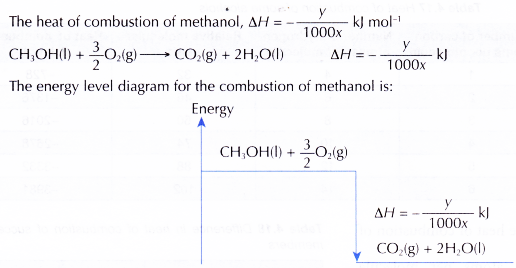
2. Similarly, the heats of combustion of ethanol, propan-1 -ol and butan-1 -ol can be calculated.
Discussion:
- Combustion of alcohol is an exothermic reaction, thus heat is given out.
- An alcohol is a compound containing carbon, hydrogen and oxygen.
- The heat of combustion of each alcohol depends on the number of carbon and hydrogen atoms in the molecular formula of the alcohol molecule.
- The higher the number of carbon and hydrogen atoms per alcohol molecule, the higher is the heat of combustion.
- The heat of combustion obtained from the experiment is always less than the theoretical value. This is because:
(a) The combustion of alcohol is not always complete. Instead of carbon dioxide and water being formed, carbon and carbon monoxide might be formed due to the incomplete combustion.
(b) Some heat is lost to the surroundings during combustion.
(c) A small quantity of the alcohol has been evaporated. - The following precautions need to be taken in order to obtain a more accurate result.
(a) The flame from the burning of alcohol must always touch the base of the copper can.
(b) A thin copper can is used. Copper is a good conductor of heat, thus it can transfer the heat given out during the combustion of alcohol to the water.
(c) A wire gauze is not used in the experiment because it might absorb some of the heat given out during the combustion.
(d) When the flame has been put out, the spirit lamp must be weighed immediately because alcohols evaporate easily.
(e) The water in the copper can must be stirred throughout the whole experiment to ensure that the temperature of the water is uniform.
(f) A windshield is used to shield the flame from air currents. Air currents accelerate heat loss to the surroundings.
Conclusion:
The heat of combustion increases as the number of carbon and hydrogen atoms per alcohol molecule increases. Thus, the hypothesis is accepted.
How to calculate heat combustion example problems with solutions
1. Complete combustion of 1 mole of butan-l-ol, C4H9OH produces 2678 kJ of heat. Calculate the mass of butan-l-ol needed to burn completely in excess oxygen in order to raise the temperature of 500 cm3 of water by 35°C.
[Specific heat capacity of water: 4.2 J g-1 °C-1; density of water: 1 g cm-3]
Solution:

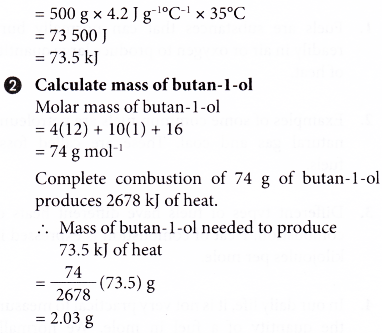
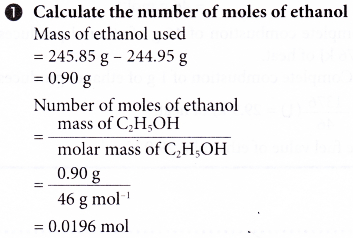
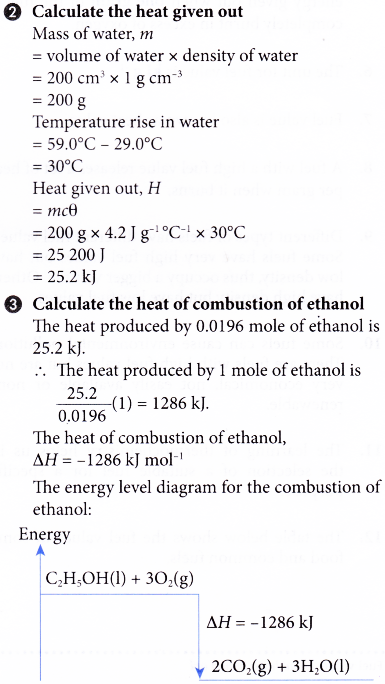
2. An experiment is carried out to determine the heat of combustion of ethanol, C2H5OH. The results of the experiment are shown below.
Volume of water used = 200 cm3
Initial temperature of water = 29.0°C
Highest temperature of water = 59.0°C
Mass of spirit lamp and ethanol before combustion = 245.85 g
Mass of spirit lamp and ethanol after combustion = 244.95 g
Based on the results, calculate the heat of combustion of ethanol and construct the energy level diagram for the complete combustion of ethanol.
[Specific heat capacity of water: 4.2 J g-1 °C-1; density of water: 1 g cm-3; relative atomic mass: H, 1; C, 12; O,16]
Solution: