What does electrochemical series mean?
The Electrochemical Series:
- The electrochemical series is an arrangement of metals based on the tendency of each metal atom to donate electrons.
- The greater the tendency to donate electrons, the more electropositive is the metal and the higher it is in the electrochemical series.
- The electrochemical series can be constructed based on
(a) the potential difference between two metals.
(b) the ability of a metal to displace another metal from its salt solution.
Constructing the electrochemical series based on the potential difference between two metals
- In a voltaic cell, two different metals are used to create a potential difference, which is shown by the reading of the voltmeter.
The greater the voltage produced by the cell, the further the two metals are in the electrochemical series. - When a voltmeter shows a positive reading, the metal that is connected to the negative terminal of the voltmeter will be the negative terminal of the cell. This metal is situated at a higher position in the electrochemical series than the other metal.
Constructing the electrochemical series based on the ability of a metal to displace another metal from its salt solution
- A metal which is situated at a higher position in the electrochemical series is able to displace a metal below it in the series from its salt solution. This happens because metals with higher positions have a greater tendency to form positive ions.
- When a piece of zinc metal is placed in copper(II) sulphate solution, the following is observed.
• Part of the zinc plate dissolves.
• A brown solid forms on the surface of the remaining part of the zinc plate.
• The blue colour of the copper(II) sulphate solution becomes colourless.
The brown solid is copper metal. The solution turns colourless because zinc displaces copper from the copper(II) sulphate solution. Therefore, zinc is above copper in the electrochemical series.
This reaction is called the metal displacement reaction. The equation for the reaction is
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s) - When a piece of zinc metal is placed in magnesium sulphate solution, no reaction occurs. Therefore, zinc is below magnesium in the electrochemical series.
People also ask
- Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot?
- Analysing the electrolysis of molten compounds
- Analysing the Electrolysis of Aqueous Solutions
- How does a voltaic cell work?
- How is electrolysis used in the industry?
Importance of Electrochemical Series
The electrochemical series can be used to
(a) determine the terminals of a voltaic cell
- The metal which is situated at a higher position in the electrochemical series will be the negative terminal while the metal which is situated at a lower position in the electrochemical series will be the positive terminal.
- For example, in a zinc-copper cell, the zinc electrode acts as the negative terminal while the copper electrode acts as the positive terminal.
(b) compare the standard cell voltages of voltaic cells
- The further apart the metals are in the electrochemical series, the bigger is the voltage.
- For example, magnesium and copper are further apart than zinc and copper, so a magnesium- copper cell has a bigger voltage than a zinc- copper cell.
(c) predict the ability of a metal to displace another metal from its salt solution
- A metal which is situated at a higher position in the electrochemical series is able to displace a metal below it in the series from its salt solution.
- For example, zinc can displace metals such as iron, lead and copper from their salt solutions because zinc is above these metals in the electrochemical series. However, zinc cannot displace metals such as sodium or aluminium because both of these metals are above zinc in the electrochemical series.
Importance of Electrochemical Industries
The importance of electrochemical industries in our daily life is as follows:
- Chlorine can be produced in bulk by electrolysis.
- Very reactive metals such as aluminium and sodium are extracted by electrolysis.
- The electrorefining industry produces very pure metals.
- Electroplating such as tin-plating and chromium-plating are widely used to prevent the rusting of iron and steel.
- Cutlery and tea sets are often silver-plated. The layer of silver makes the objects beautiful and shiny.
- The industrial manufacture of batteries produces various types of cells and batteries in different shapes and sizes.
The pollution caused by the industrial processes involving electrochemical industries is as follows:
- The electroplating industries produce pollutants such as poisonous heavy metal ions, chemicals which alter the pH of local water sources and poisonous cyanide ions.
- The use of mercury electrodes in electrolysis can cause air and water pollution.
- The hydrogen fluoride gas that is formed during the extraction of aluminium is very corrosive and will pollute the air.
- Used mercury cells are a danger to the environment.
Chemical wastes from electrochemical industries may be poisonous and can endanger our health. Therefore, these wastes must be treated and disposed of in a safe and orderly manner.
Electrochemical Series Experiment 1
Aim: To construct the electrochemical series based on the potential difference between two metals.
Problem statement: How can the electrochemical series be constructed based on the potential difference between two metals?
Hypothesis: The further apart the pair of metals in the electrochemical series, the greater is their potential difference.
Variables:
(a) Manipulated variable: Pairs of metals
(b) Responding variable: Potential difference
(c) Controlled variables: Type of electrolyte, concentration of electrolyte, copper electrode
Operational definition: The reading of the voltmeter is the potential difference between two metals.
Materials: 0.1 mol dm-3 copper(II) sulphate solution, copper strip, lead strip, iron nail, zinc strip, magnesium ribbon, aluminium strip and sandpaper.
Apparatus: Voltmeter, 250 cm3 beaker and connecting wires with crocodile clips.
Procedure:
- Two-thirds of a beaker is filled with copper(II) sulphate solution.
- A magnesium ribbon and a copper strip are cleaned with sandpaper.
- The magnesium and copper electrodes are placed into the copper(II) sulphate solution.
- The electrodes are connected to the voltmeter as shown in.
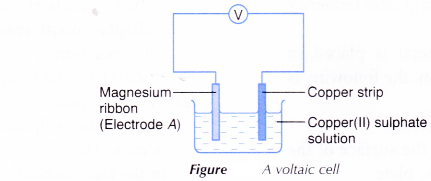
- The reading of the voltmeter is recorded.
- The metal strip which becomes the negative terminal is determined and recorded.
- Steps 1 to 6 are repeated using other metals to replace the magnesium ribbon as electrode A.
Results:
| Pair of metals | Potential difference (V) | Negative terminal of the cell |
| Magnesium and copper | 2.7 | Magnesium |
| Aluminium and copper | 2.0 | Aluminium |
| Zinc and copper | 1.1 | Zinc |
| Iron and copper | 0.8 | Iron |
| Lead and copper | 0.5 | Lead |
| Copper and copper | 0.0 | – |
Discussion:
- Figure shows the potential differences of different voltaic cells using different metals as electrodes.
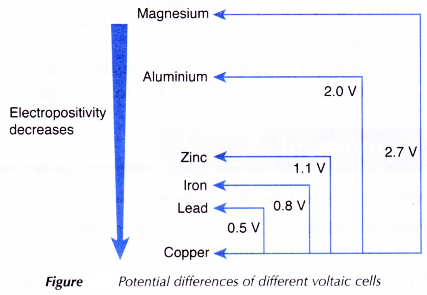
- In all the cells, the copper plate acts as the positive terminal of the cell. Therefore, copper is situated at the lowest position among the metals in the electrochemical series.
- When the magnesium ribbon is connected to the copper plate, the voltmeter shows the highest reading. This shows that magnesium is situated furthest from copper in the electrochemical series.
- No current will flow if both the electrodes are made of copper metal.
Conclusion:
The descending order of the electropositivity of the metals in the electrochemical series is magnesium, aluminium, zinc, iron, lead, copper. The hypothesis is accepted.
Electrochemical Series Experiment 2
Aim: To construct the electrochemical series based on the ability of a metal to displace another metal from its salt solution.
Problem statement: How can the electrochemical series be constructed based on the ability of a metal to displace
another metal from its salt solution?
Hypothesis: A metal which is situated at a higher position in the electrochemical series is able to displace a metal below it in the series from its salt solution.
Variables:
(a) Manipulated variables: Pairs of metal and salt solution used
(b) Responding variable : Precipitation of metal or change in colour of solution
(c) Controlled variable : Concentration of salt solution
Operational definition: Metal displacement reaction occurs when there is a deposition of metal or change in colour of solution.
Materials: 1 mol dm-3 copper(II) nitrate solution, 1 mol dm-3 lead(II) nitrate solution, 1 mol dm-3 iron(II) nitrate solution, 1 mol dm-3 zinc nitrate solution, 1 mol dm-3 magnesium nitrate solution, copper strip, lead strip, iron nail, zinc strip, magnesium ribbon and sandpaper.
Apparatus: Test tubes and test tube rack.
Procedure:
- Five test tubes are filled with copper(II) nitrate solution respectively until they are half full.
- A piece of copper strip, lead strip, iron nail, zinc strip, and magnesium ribbon are cleaned respectively with sandpaper and dropped into each of the test tubes.
- The reactions (if any) are allowed to take place for five minutes.
- Any change in the colour of the solutions and whether any metals are deposited are observed.
- Steps 1 to 4 are repeated using lead(II) nitrate solution, iron(II) nitrate solution, zinc nitrate solution and magnesium nitrate solution respectively to replace copper(II) nitrate solution.
- The results of the experiment are recorded in a table.
Results:
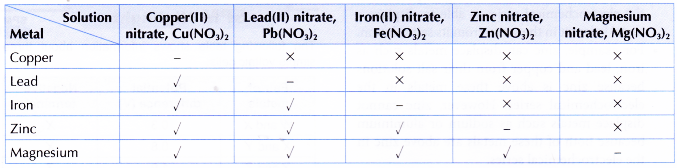
Discussion:
- From the results, magnesium must be placed at the highest position in the electrochemical series because it reacts with all the salt solutions. On the other hand, copper must be placed at the lowest position in the electrochemical series because it does not react with any of the salt solutions.
- The positions of the metals in the electrochemical series are as follows:

- Magnesium can displace copper, lead, iron and zinc because these metals are below magnesium in the electrochemical series.
- Copper cannot displace lead, iron, zinc and magnesium because these metals are above copper in the electrochemical series.
Conclusion:
The descending order of electropositivity of the metals in the electrochemical series is magnesium, zinc, iron, lead, copper. The hypothesis is accepted.
Electrochemical Series Experiment 3
Aim: To confirm the predictions of the metal displacement reaction.
Problem statement: How to confirm the predictions of the metal displacement reaction?
Hypothesis: If a metal can displace another metal from its salt solution, then there is a change of colour in the solution or a precipitation of metal.
Variables:
(a) Manipulated variables: Pairs of metal and salt solution used
(b) Responding variable: Precipitation of metal or change in colour of solution
(c) Controlled variable: Concentration of salt solution
Materials: 0.1 mol dm-3 silver nitrate solution, 0.1 mol dm-3 potassium nitrate solution, 0.1 mol dm-3 tin(II) nitrate solution, copper strip, magnesium ribbon, iron nail and sandpaper.
Apparatus: Test tubes and test tube rack.
Procedure:
- Silver nitrate solution, potassium nitrate solution and tin(II) nitrate solution are poured into three separate test tubes, X, Y and Z, until they are half full.
- The copper strip, magnesium ribbon and iron nail are cleaned with sandpaper and placed in the different salt solutions in the test tubes as shown in Figure.
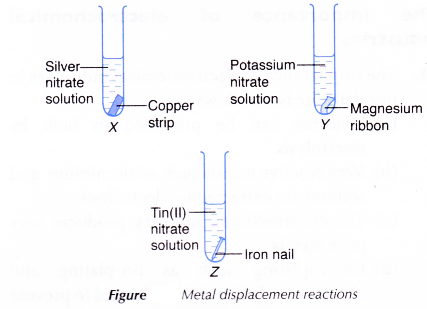
- The reactions (if any) are allowed to take place for five minutes.
- Any change in the colour of the solutions and whether any metals are deposited are observed.
Results:
| Test tube | Prediction | Observation |
| X | Copper is above silver in the electrochemical series. Thus, copper will displace silver from silver nitrate solution. | The copper strip dissolves. A shiny grey solid is deposited. The colourless solution turns blue. |
| Y | Magnesium is below potassium in the electrochemical series. Thus, magnesium will not displace potassium from potassium nitrate solution. | No visible change. |
| Z | Iron is above tin in the electrochemical series. Thus, iron will displace tin from tin(II) nitrate solution. | The iron nail dissolves. A shiny grey solid is deposited. The colourless solution turns light green. |
Discussion:
- Test tube X:
The shiny grey solid is silver metal. The colourless solution turns blue because copper(II) ions have been released into the solution. The equation for the reaction is
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
The prediction is correct. The metal displacement reaction occurs. - Test tube Y:
The prediction is correct. The metal displacement reaction does not occur. - Test tube Z:
The shiny grey solid is tin metal. The colourless solution turns light green because iron(II) ions have been released into the solution. The equation for the reaction is
Fe(s) + Sn(NO3)2(aq) → Fe(NO3)2(aq) + Sn(s)
The prediction is correct. The metal displacement reaction occurs.
Conclusion:
The ability of a metal to displace another metal from its salt solution can be predicted using the electrochemical series. The prediction can be confirmed through an experiment. The hypothesis is accepted.
