What is the effect of a catalyst on the rate of a reaction?
Effect of catalyst on the rate of reaction:
- A catalyst is a substance which can alter the rate of a chemical reaction while itself remains chemically unchanged at the end of the reaction.
- (a) Catalysts can be classified into positive catalysts and negative catalysts (inhibitors).
(b) A positive catalystis a catalyst that increases the rate of a reaction.
(c) A negative catalyst is a catalyst that decreases the rate of a reaction.
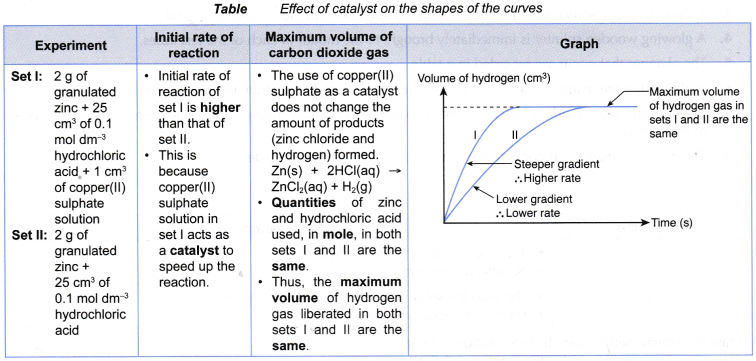
Table shows a few examples of catalysed reactions.
| Type of reaction | Catalyst used | Effect of catalyst on the rate of reaction |
| Reaction between zinc and sulphuric acid Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) | Copper(II) sulphate solution | Rate of reaction increases. (Positive catalyst) |
| Decomposition of hydrogen peroxide solution 2H2O2(aq) → 2H2O(I) + O2(g) | Manganese(IV) oxide/ lead(II) oxide | Rate of reaction increases. (Positive catalyst) |
| Decomposition of sodium chlorate(l) solution 2NaOCl(aq) → 2NaCl(aq) + O2(g) | Manganese(IV) oxide | Rate of reaction increases. (Positive catalyst) |
| Decomposition of hydrogen peroxide solution 2H2O2(aq) → 2H2O(l) + O2(g) | Propane-1,2,3-triol (glycerine) | Rate of reaction decreases. (Negative catalyst) |
- A catalyst does not change the quantity of the products formed. It only alters the rate of the reaction.
- An example of the effect of catalyst on the rate of reaction is shown in table.
People also ask
- What is the rate of the reaction?
- How do you calculate the reaction rate?
- What factors affect the rate of a reaction?
- How does the surface area affect the rate of reaction?
- Explain the effect of concentration on the rate of reaction?
- How does the temperature affect the rate of a chemical reaction?
- What is the collision theory in chemistry?
- How does the collision theory affect the rate of reaction?
Characteristics of catalysts:
Catalysts exhibit the following characteristics:
- Catalyst remains chemically unchanged at the end of a reaction. It means that the quantity and chemical composition of the catalyst remain unchanged at the end of the reaction.
- Catalyst alters the rate of a chemical reaction. Positive catalyst increases the rate of reaction. Negative catalyst decreases the rate of reaction.
- Catalyst does not change the quantity of products formed. The amount of products remains the same with or without the catalyst.
- Catalyst is specific in its action. It can only catalyse a particular reaction but not other reactions.
- Only a small amount of catalyst is needed to achieve a big increase in the rate of reaction.
- An increase in the amount of catalyst used will cause only a very slight increase in the rate of reacton. Hence, it is not necessary to use a large amount of catalyst in a reaction.
- Finely divided (powdered) catalyst is more effective than lump catalyst.
- Catalyst used can be in the solid, liquid, gas or aqueous state. A solid catalyst may undergo physical changes. For example, crystals may turn into powder during the reaction.
Most catalysts are transition elements or compounds of transition elements such as iron, platinum, nickel, vanadium(V) oxide, copper(II) sulphate and manganese(IV) oxide.
Effect of pressure on the rate of‘reaction
- For reactions with reactants in the form of solid, liquid and aqueous solution, the rates of the reactions do not depend on the pressure.
- Only for those reactions that involve gaseous reactants, the rates of the reactions will increase when the pressure increases or vice versa.
- An example is illustrated by the combustion of petrol in the car engine as explained below:
- Petrol is a liquid mixture of hydrocarbons.
- In the car engine, the mixture of petrol vapour and air is compressed to a high pressure so that the mixture can be ignited rapidly by the spark plug.
- The high pressure results in a high rate of combustion of petrol.
Effect of catalyst on rate of reaction experiment 1
Aim: To investigate the effect of catalyst on the rate of reaction.
Problem statement: How does a catalyst affect the rate of reaction?
Hypothesis: The presence of a catalyst will increase the rate of reaction.
Variables:
(a) Manipulated variable : Presence or absence of a catalyst
(b) Responding variable : Rate of reaction
(c) Controlled variables : Volume and concentration of hydrogen peroxide solution, temperature
Operational definition:
The decomposition of hydrogen peroxide is fast if the wooden splint rekindles brightly and rapidly. The decomposition of hydrogen peroxide is slow if the wooden splint glows dimly and slowly.
Materials: 20-volume hydrogen peroxide solution, manganese(IV) oxide powder, filter paper, wooden splints.
Apparatus: Test tubes, retort stands and clamps, 10 cm3 measuring cylinder, filter funnel, 150 cm3 beaker, spatula, electronic balance.
Procedure:
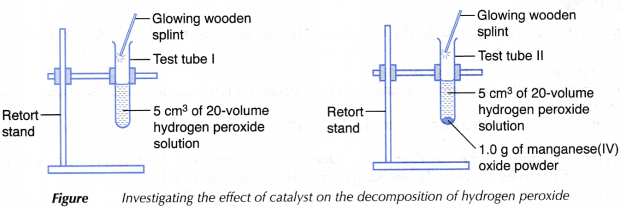
- Two test tubes are labelled I and II respectively.
- 5 cm3 of 20-volume hydrogen peroxide solution is measured and poured separately into test tubes I and II respectively.
- 1.0 g of manganese(IV) oxide powder is weighed and added to the solution in test tube II.
- A glowing wooden splinter is immediately brought to the mouth of each of the test tubes.
- The changes that occur are recorded in a table.
- At the end of the reaction, the mixture in test tube II is filtered to separate the manganese(IV) oxide powder.
- The manganese(IV) oxide powder obtained is washed with a little distilled water and pressed between sheets of filter paper.
- The dry manganese(IV) oxide powder is weighed and its mass is recorded.
Results:
| Test tube | Observation |
| I | The wooden splint glows dimly and slowly. No effervescence occurs. |
| II | The wooden splint rekindles brightly and rapidly. Effervescence occurs. |
Mass of manganese(IV) oxide before reaction = 1.0 g
Mass of manganese(IV) oxide after reaction = 1.0 g
Inferences:
- Rate of decomposition of hydrogen peroxide increases in the presence of manganese(IV) oxide powder.
- Mass of manganese(IV) oxide powder remains unchanged during the reaction.
- Manganese(IV) oxide acts as a catalyst.
Discussion:
- (a) Oxygen gas is liberated during the decomposition of hydrogen peroxide.
2H2O2(aq) → 2H2O(l) + O2(g)
(b) The liberation of oxygen gas can be confirmed if the wooden splint rekindles brightly or continues to glow. - Manganese(IV) oxide acts as a (positive) catalyst to increase the rate of decomposition of hydrogen peroxide while itself remains chemically unchanged at the end of the reaction. Hence, its mass remains unchanged at the end of the reaction.
Conclusion:
The presence of a (positive) catalyst increases the rate of a reaction. Hence, the hypothesis can be accepted.
Effect of catalyst on rate of reaction experiment 2
Aim: To investigate the effect of the amount of catalyst on the rate of reaction.
Problem statement: How does the amount of a catalyst affect the rate of a reaction?
Hypothesis: When the amount of a catalyst used increases, the rate of reaction also increases.
Variables:
(a) Manipulated variable : Amount/mass of catalyst
(b) Responding variable : Rate of reaction
(c) Controlled variables : Temperature, volume and concentration of hydrogen peroxide solution
Operational definition:
The curve for the graph of volume of gas liberated against time with a higher gradient indicates a higher rate of reaction.
Materials: 2-volume hydrogen peroxide solution, manganese(IV) oxide powder.
Apparatus: 50 cm3 measuring cylinder, 150 cm3 conical flask, stopper fitted with a delivery tube, burette, retort stand and clamp, basin, electronic balance, stopwatch, spatula, beaker.
Procedure:
- A burette is filled with water until it is full. The burette is inverted over water in a basin and clamped vertically using a retort stand.
- The water level in the burette is adjusted and the initial burette reading is recorded.
- 50 cm3 of 2-volume hydrogen peroxide solution is measured using a measuring cylinder and poured into a conical flask.
- 0.2 g of manganese(IV) oxide powder is weighed using an electronic balance and poured carefully into the conical flask.
- The conical flask is then closed immediately with a stopper fitted with a delivery tube directed to the burette, as shown in Figure. At the same time, a stopwatch is started immediately.
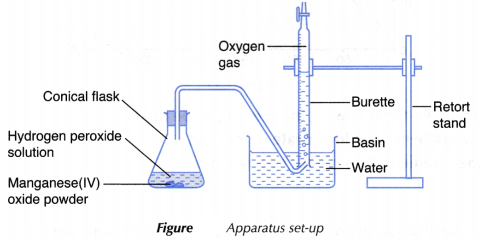
- The conical flask with its contents is shaken slowly and the volume of oxygen gas collected in the burette is recorded at regular time intervals of 30 seconds for 5 minutes.
- Steps 1 to 6 are repeated using 0.8 g of manganese(IV) oxide powder to replace the 0.2 g of manganese(IV) oxide powder.
- The results are tabulated.
Results:
Set I: Using 0.2 g of manganese(IV) oxide powder
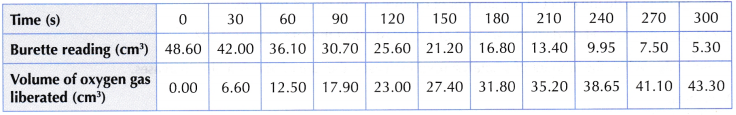
Set II: Using 0.8 g of manganese(IV) oxide powder
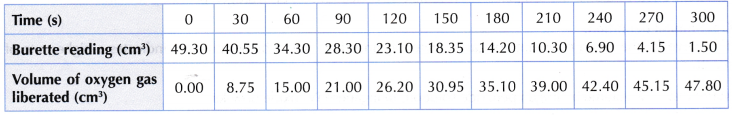
Interpreting data:
- Based on the results obtained, the graphs of the volume of oxygen gas liberated against time for both sets I and II are plotted on the same axes.
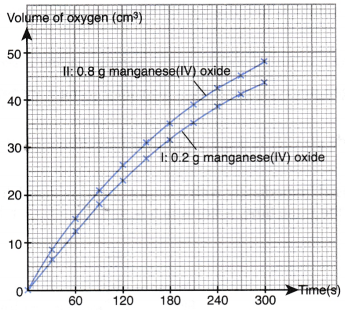
- Based on the graphs plotted, the following inferences can be made.
- Curve I has a lower gradient, thus lower rate of reaction.
- Curve Il has a higher gradient, thus higher rate of reaction.
Discussion:
- The amount of manganese(IV) oxide powder (catalyst) used in set II is four times the amount of manganese(IV) oxide powder used in set I.
- Based on the inferences made, it can be deduced that:
As the amount of manganese(IV) oxide powder (catalyst) used increases, the rate of reaction also increases. - There is only a very slight increase in the rate of reaction when using 4 times more manganese(IV) oxide powder as a catalyst.
- (a) If the reaction is allowed to proceed until all the hydrogen peroxide is completely decomposed, the graphs in Figure will be obtained.
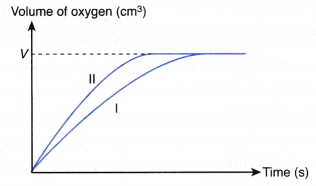
(b) (i) The graphs show that the maximum volume of oxygen gas liberated in both sets I and II are the same, that is, V cm3.
(ii) This is because the quantities of hydrogen peroxide solution used, in mole, in both sets I and II are the same.
(iii) The manganese(IV) oxide powder as a catalyst does not affect the amount of products formed.
Conclusion:
An increase in the amount of catalyst used will increase the rate of reaction. Hence, the hypothesis can be accepted.
Note: There is only a very slight increase in the rate of reaction when the amount of catalyst increases.
