Why does Diffusion take place
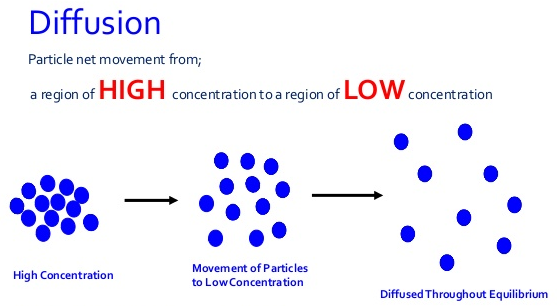
Diffusion : The spreading out and mixing of a substance with another substance due to the motion of its particles is called diffusion.
Diffusion is a property of matter which is based on the motion of its particles.
Diffusion is fastest in gases because the particles in gases move very rapidly. The diffusion is slowest in solids because the particles in solids do not move much.
The rate of diffusion increases on increasing the temperature of the diffusing substance. This is because when the temperature of a substance is increased by heating, its particles gain kinetic energy and move more rapidly and this increase in the speed of the particles of a substance increases the rate of diffusion.
 Diffusion in gases
Diffusion in gases
Diffusion in gases is very fast. This is because the particles in gases move very quickly in all directions.
Ex. When we light an incense stick (agarbatti) in a corner of our room, its fragrance spreads in the whole room very quickly. The fragrance of burning incense stick spreads all around due to the diffusion of its smoke into the air.
Ex. When someone opens a bottle of perfume in one corner of a room, its smell spreads in the whole room quickly. The smell of perfume spreads due to the diffusion of perfume vapours into air.
Diffusion in liquids
Diffusion in liquids is slower than that in gases. This is because the particles in liquids move slower as compared to the particles in gases.
Ex. The spreading of purple colour of potassium permanganate into water, on its own, is due to the diffusion of potassium permanaganate particles into water
Ex. The spreading of blue colour of copper sulphate into water, on its own, is due to the diffusion of copper sulphate particles into water.
The rate of diffustion in liquids is much faster than that in solids because the patricles in a liquid move much more freely, and have greater spaces between them as compared to particles in the solids.
Diffusion in solids
Diffusion in solids in a very, very slow process.
Ex. If we write something on a blackboard and leave it uncleaned for a considerable period of time we will find that it becomes quite difficult to clean the blackboard afterwards. This is due to the fact that some of the a particles of chalk have diffused into the surface of blackboard.
Ex. If two metal blocks are bound together tightly and kept undisturbed for a few years, then the particles of one metal are found to have diffused into the other metal.
