What determines a Strong Base and a Weak Base
Strong Bases and Weak Bases :
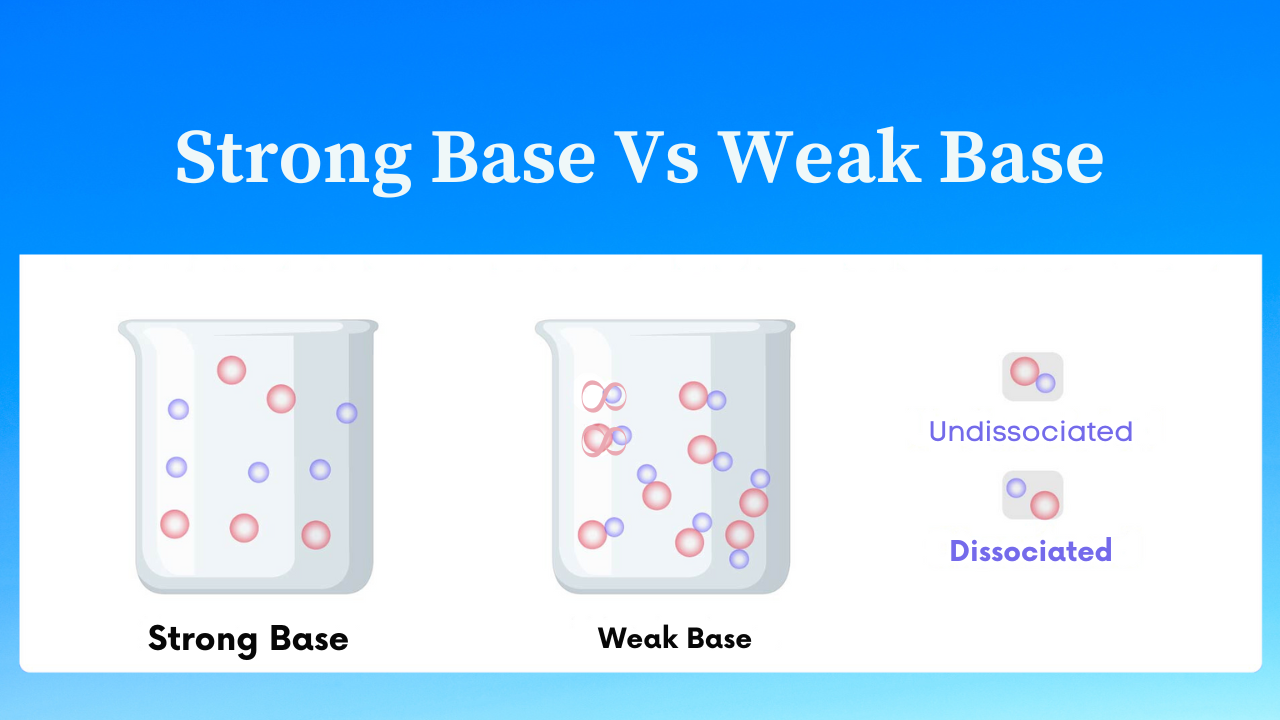
The strength of a base is determined by the amount of hydroxide ions (OH–) that the base provides when dissolved in water.
Some of the bases, when dissolved in water, get almost completely dissociated to provide hydroxide ions. These bases are called strong bases. (Bases soluble in water are also called alkalis.) For example, sodium hydroxide and potassium hydroxide are strong bases.
But there are bases which, when dissolved in water, get only partially dissociated to give hydroxide ions. These are weak bases. For example, magnesium hydroxide and ammonium hydroxide are weak bases.
Acidity of a Base :
The acidity of a base is defined as the number of hydroxyl (OH) groups present in a molecule of the base.
In each molecule of NaOH, KOH and NH4OH only one hydroxyl group is present. Therefore, the acidity of all these bases is 1.
In Ca(OH)2 and Ba(OH)2 there are two hydroxyl groups present in each molecule. Hence, their acidity is 2.
Similarly, the acidity of Fe(OH)3 and Al(OH)3 is 3.
The base containing one hydroxyl group in a molecule is said to be mono acidic base, that containing two hydroxyl groups is called diacidic base, and that containing three hydroxyl groups is called triacidic base. Thus, NaOH, Ca(OH)2 and Fe(OH)3 are monoacidic, diacidic and triacidic bases respectively.
People also ask
- What is the definition of an acid and a base?
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution
