What is the definition of Indicator in chemistry
Indicator
Indicators are substances which show a change in colour when brought in contact with acids and bases.
The most common indicator used in the laboratory is the litmus. It is extracted from lichens and is available either in the form of solutions or in the form of strips of paper known as litmus paper. Litmus paper is either red or blue in colour. It changes colour depending upon whether the compound is an acid or a base. Blue litmus paper turns red under acidic conditions, and red litmus paper turns blue under basic conditions.
The solutions that do not show change in colour in any of the indicators are neither acidic nor basic. These substances, as you already know, are called neutral substances. Some examples of neutral substances are water, sodium chloride, sugar, etc.
Litmus, methyl orange and phenolphthalein are some of the most commonly used acid-base indicators that change colour as follows.
Indicator | Acid solution | Basic solution | Neutral solution |
Blue litmus solution | Red | No change in colour | No change in colour |
Red litmus solution | No change in colour | Blue | No change in colour |
Methyl orange | Red | Yellow | Orange |
Phenolphthalein | Colourless | Red | Colourless |
Litmus : It is a natural dye made from small plants called lichens. Blue and red litmus solutions are prepared from two different varieties of lichens.
Litmus paper : Blue or red litmus paper is prepared by dipping a strip of filter paper in blue or red litmus solutions. The paper is then removed from the solution and dried.
Blue litmus paper turns red in an acidic solution and red litmus paper blue in a basic solution.
Phenolphthalein : It is a colourless compound. An alcoholic solution of phenolphthalein is used as an indicator. It is colourless in an acidic solution, but becomes pink (red) in basic solution:
Methyl orange : A very small amount of solid methyl orange is dissolved in hot water and filtered. The filtrate is used as an indicator. It turns red in acid solutions and yellow in basic solutions.
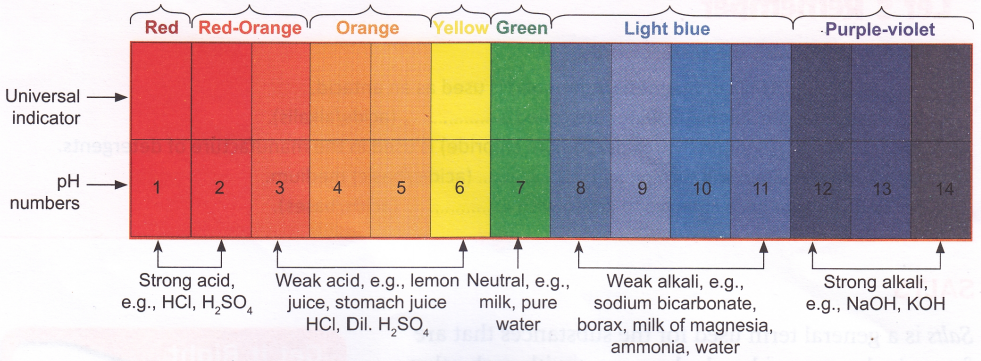
Universal Indicators
Acids or bases can be either strong or weak. Scientists have made it very easy to know the strength of an acid or a base. Different strengths have been given what is known as a pH number which ranges from 1 to 14. One is considered as strongly acidic and 14, strongly basic; 7 is neutral. You can measure pH of a solution using a Acids or bases can be either strong or weak. Scientists have made it very easy to know the strength of an acid or a base. Different strengths have been given what is known as a pH number which ranges from 1 to 14. One is considered as strongly acidic and 14, strongly basic; 7 is neutral. You can measure pH of a solution using a universal indicator.
A universal indicator is a mixture of indicators so chosen that it gives a different colour for different pH values. The indicator can be used as a liquid or can be soaked into paper. This paper is called pH paper.
When a pH paper is dipped in an acid or a base, the colour obtained can be matched with the chart given below.
Natural indicators :
Some useful Natural indicators are discussed below.
(i) Turmeric juice It is yellow in colour. It remains yellow in acidic or neutral solutions but turns deep brown in a basic solution.
(ii) Red-cabbage juice Itself purple in colour, it turns red in an acid solution, but green in a basic solution.
Indicator solutions can be prepared by boiling coloured parts of the plant, such as petals, in i water for sometime and straining out the plant part. This solution gives a different colour in acidic and basic solutions.
For example, red cabbage juice will change to deep red with acids, to purple with neutrals, and to green and yellow with bases. Onion juice also shows similar colour changes. You can directly put some rose petals in a dish containing vinegar or lemon juice and a few petals in soap solution and see the difference in colour.
The household indicators may be used to test whether some of the substances of daily use as listed below are acidic or basic.
| Acidic substances | Basic substance |
| Vitamine C tablets (ascorbic acid) Lemon juice Orange juice Tomato juice Vinegar | Antacids Toothpaste Soap solution Washing soda solutions |
Olfactory indicators :
There are substances like onion juice, vanilla essence and clove oil which by change of their smell indicate whether the sample solution is acidic or basic. These are called olfactory indicators.
You have learnt that in neutralization reactions an acid and a base react to produce salt and water. For example, the neutralization reaction between NaOH and HCl gives the salt NaCl and water.
NaOH + HCl → NaCl + H2O
Thus, a salt may be defined as follows.
A salt is a compound formed by the reaction of an acid with a base in which the hydrogen of the acid is replaced by the metal.
In polybasic acids, more than one hydrogen atoms are present in a molecule. These hydrogen atoms can be replaced partially or completely. So, two kinds of salts are possible.
(i)
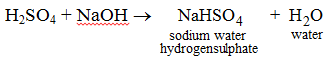
Here, partial replacement of hydrogen atoms from H2SO4 has resulted in the formation of sodium hydrogensulphate.
(ii)
![]()
Here, complete replacement of hydrogen atoms from H2SO4 has resulted in the formation of sodium sulphate. NaHSO4 and Na2SO4 represent two kinds of salts.
Activity
Aim: To see the effect of a natural indicator
Materials needed: Turmeric powder, alcohol/water, soap solution, and beaker Method: –
1. Mix 1/4th teaspoon of turmeric (haldi) powder in 1/4 cup of alcohol in a small beaker. You can also mix it with water but turmeric dissolves better in alcohol.
2. Prepare a soap solution in another beaker by dissolving a small piece of washing soap in water.
3. Add a few drops of the turmeric-alcohol solution to the soap solution.
Observation: The soap solution turns red.
Conclusion: Soap is a base, and the compound (called curcumin) that gives turmeric its yellow
colour reacts with soap (a base) to form a red compound.
