What is the definition of an acid and a base?
What is the Arrhenius definition of an acid and a base?
What are acids?
- The Swedish chemist, Arrhenius proposed the following definition of an acid.
An acid is a substance which ionises or dissociates in water to produce hydrogen ions, H+. - For example, hydrochloric acid, HCl(aq) is a solution of hydrogen chloride in water, obtained by dissolving pure hydrogen chloride gas in water.
HCl(g) → HCl(aq)
As the gas dissolves in water, the hydrogen chloride molecule reacts with water and ionises to produce hydrogen ion, H+.
HCl(aq) → H+(aq) + Cl–(aq)
The hydrogen ion then attaches itself to a water molecule to form the hydroxonium ion, H3O+.
H+(aq) + H2O(l) → H3O+(aq)
Hence, the overall equation for the ionisation of hydrogen chloride is given below.
HCl(aq) + H2O(1) → H3O+(aq) + Cl–(aq)
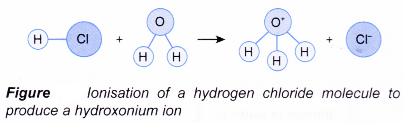
- For the sake of convenience, the term ‘hydrogen ion is used to replace ‘hydroxonium ion and H+(aq) is used in place of H3O+(aq).
- Hence, the ionisation of hydrochloric acid in water can be represented as:
- Other acids ionise similarly in water. Examples:
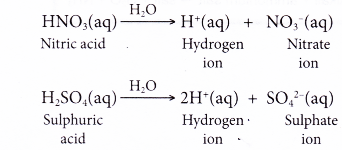
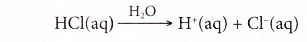
- Hydrochloric acid is known as a monoprotic acid. This acid contains only one ionisable hydrogen atom, producing only one hydrogen ion (proton) per molecule of acid.
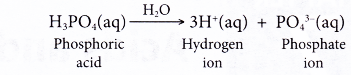
- Polyprotic acids can produce more than one hydrogen ion per molecule of acid. Sulphuric acid is a diprotic acid, whereas phosphoric acid is a triprotic acid.
- Basicity of an acid is the number of ionisable hydrogen atoms per acid molecule.
- A number of non-metal oxides react with water to produce acidic solutions which contain hydrogen ions and turn blue litmus paper red. They are called acidic oxides.
(a) Carbon dioxide reacts with water to form carbonic acid.
CO2(g) + H2O(l) → H2CO3(aq)
(b) Sulphur trioxide reacts with water to form sulphuric acid.
SO3(g) + H2O(l) → H2SO4(aq)
(c) Dinitrogen pentoxide reacts with water to form nitric acid.
N2O5(g) + H2O(l) → 2HNO3(aq) - Not all non-metal oxides are acidic oxides. Only those that are able to react with water can produce acidic solutions. For example, carbon monoxide does not react with water. Therefore, carbon monoxide is classified as a neutral oxide.
- Acids are classified into two groups, mineral acids and organic acids.
- Mineral acids are obtained frdm minerals, whereas organic acids are extracted from animal and plant materials.
Table 1 and Table 2 show some examples of mineral and organic acids.
| Mineral acid | |
| Name | Formula |
| Carbonic acid | H2CO3 |
| Hydrochloric acid | HCl |
| Hydrochlorous acid | HClO |
| Nitrous acid | HNO2 |
| Nitric acid | HNO3 |
| Sulphurous acid | H2SO3 |
| Sulphuric acid | H2SO4 |
| Phosphoric acid | H3PO4 |
| Organic acid | |
| Name | Formula |
| Methanoic acid | HCOOH |
| Ethanoic acid | CH3COOH |
| Propanoic acid | C2H5COOH |
| Ascorbic acid | C6H8O5 |
| Citric acid | C6H6O7 |
| Lactic acid | C3H6O3 |
| Malic acid | C4H6O5 |
| Ethanedioic acid | H2C2O4 |
People also ask
- What is the definition of an acid in chemistry?
- What is the definition of a base in chemistry?
- Classification of Acids
- Preparation of Acids
- What are the chemical properties of an acid?
- General Properties of Acids
- Uses of Acids
- Preparation of Bases
- General Properties of Bases
- What determines a Strong Base and a Weak Base
- What are the uses of Bases
- How can we measure the strength of acids and alkalis?
- How to calculate concentration of acids and alkalis?
- How do you prepare a standard solution?
- What is meant by a neutralization reaction?
- How does titration determine concentration?
- Relationship between pH values and molarity of acids and alkalis
- Concept of the pH Scale
- Role of pH in everyday life
- What is the pH of a salt solution
What are bases?
- Arrhenius’ definition of a base:
A base is a substance which ionises in water to produce ydroxide ions, OH–. - Bases are divided into two categories, ionic bases and covalent bases.
- Ionic bases consist of metal oxides and metal hydroxides. Metal oxides contain oxide ions, O2- and metal hydroxides contain hydroxide ions, OH–.
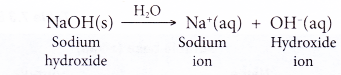
- When a soluble metal hydroxide such as sodium hydroxide dissolves in water, it ionises to release the hydroxide ion.
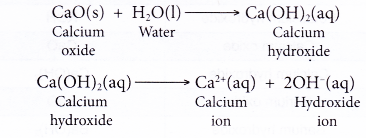
- When a soluble metal oxide dissolves in water, it reacts with water to form the hydroxide ion as one of its products. For example, calcium oxide reacts with water to form calcium hydroxide, which then ionises to produce hydroxide ion.
- Insoluble metal oxides and metal hydroxides are classified as bases because they satisfy the alternative definition of a base.
A base is a substance that reacts with an acid to form a salt and water only. - (a) For example, magnesium hydroxide reacts with hydrochloric acid to form the salt magnesium chloride and water.
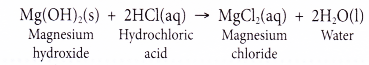
(b) On the other hand, copper(II) ‘oxide reacts with nitric acid to produce the salt copper(II) nitrate and water.
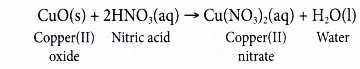
- The most common covalent base is ammonia, NH3. Ammonia solution is obtained by dissolving pure ammonia gas in water. When ammonia gas dissolves in water, it reacts with water to produce hydroxide ion.
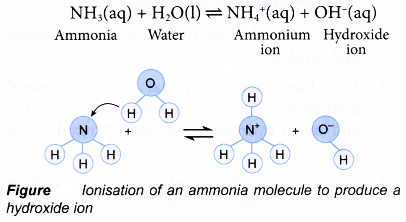
Notice that the ammonia, NH3 molecule has accepted a proton, H+ from water to form the ammonium ion, NH4+. - Bases that are soluble in water are called akalis (Figure).
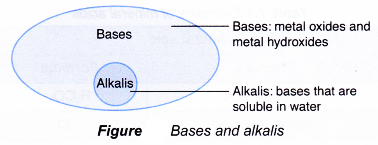
- An alkali is defined as the following.
An alkali is a base that is soluble in water and ionises to produce hydroxide ions. - According to this definition, ammonia can be classified as an alkali.
Table shows some examples of bases and alkalis.
| Soluble base (alkali) | Insoluble base | ||
| Name | Formula | Name | Formula |
| Ammonia | NH3 | Magnesium oxide | MgO |
| Sodium oxide | Na2O | Magnesium hydroxide | Mg(OH)2 |
| Sodium hydroxide | NaOH | Aluminium oxide | Al2O3 |
| Potassium oxide | K2O | Aluminium hydroxide | Al(OH)3 |
| Potassium hydroxide | KOH | Zinc oxide | ZnO |
| Calcium oxide | CaO | Zinc hydroxide | Zn(OH)2 |
| Calcium hydroxide | Ca(OH)2 | Copper(II) oxide | CuO |
| Barium oxide | BaO | Copper(II) hydroxide | Cu(OH)2 |
| Barium hydroxide | Ba(OH)2 | Lead(II) oxide | PbO |
