Anna University, Subject code – CY3151, deals with the B.E civil Engineering Semester – I Engineering Chemistry Syllabus regulation 2021 relating to affiliated institutions. From here, Students can get assistance in preparing notes to excel in academic performance.
We include every topic of the Engineering Chemistry Syllabus, to understand the subject very well. It will help you to improve your idea of syllabus of CY3151 – Engineering Chemistry Syllabus on your finger tips to go ahead in a clear path of preparation. In this following article Engineering Chemistry Syllabus, will help you, Hope you share with your friends.
If you want to know more about the syllabus of B.E Civil Engineering connected to an affiliated institution’s under four-year undergraduate degree programme. We provide you with a detailed Year-wise, semester-wise, and Subject-wise syllabus in the following link B.E Civil Engineering Syllabus Anna University, Regulation 2021.
Aim Of Concept:
- To inculcate sound understanding of water quality parameters and water treatment techniques.
- To impart knowledge on the basic principles and preparatory methods of nanomaterials.
- To introduce the basic concepts and applications of phase rule and composites.
- To facilitate the understanding of different types of fuels, their preparation, properties and combustion characteristics.
- To familiarize the students with the operating principles, working processes and applications of energy conversion and storage devices.
CY3151 – Engineering Chemistry Syllabus
Unit I: Water And Its Treatment
Water: Sources and impurities, Water quality parameters: Definition and significance of colour, odour, turbidity, pH, hardness, alkalinity, TDS, COD and BOD, flouride and arsenic. Municipal water treatment: primary treatment and disinfection (UV, Ozonation, break-point chlorination). Desalination of brackish water: Reverse Osmosis. Boiler troubles: Scale and sludge, Boiler corrosion, Caustic embrittlement, Priming &foaming. Treatment of boiler feed water: Internal treatment (phosphate, colloidal, sodium aluminate and calgon conditioning) and External treatment – Ion exchange demineralisation and zeolite process.
Unit II: Nanochemistry
Basics: Distinction between molecules, nanomaterials and bulk materials; Size-dependent properties (optical, electrical, mechanical and magnetic); Types of nanomaterials: Definition, properties and uses of – nanoparticle, nanocluster, nanorod, nanowire and nanotube. Preparation of nanomaterials: sol-gel, solvothermal, laser ablation, chemical vapour deposition, electrochemical deposition and electro spinning. Applications of nanomaterials in medicine, agriculture, energy, electronics and catalysis.
Unit III: Phase Rule And Composites
Phase rule: Introduction, definition of terms with examples. One component system – water system; Reduced phase rule; Construction of a simple eutectic phase diagram – Thermal analysis; Two component system: lead-silver system – Pattinson process. Composites: Introduction: Definition & Need for composites; Constitution: Matrix materials (Polymer matrix, metal matrix and ceramic matrix) and Reinforcement (fiber, particulates, flakes and whiskers). Properties and applications of Metal matrix composites (MMC), Ceramic matrix composites and Polymer matrix composites. Hybrid composites – definition and examples.
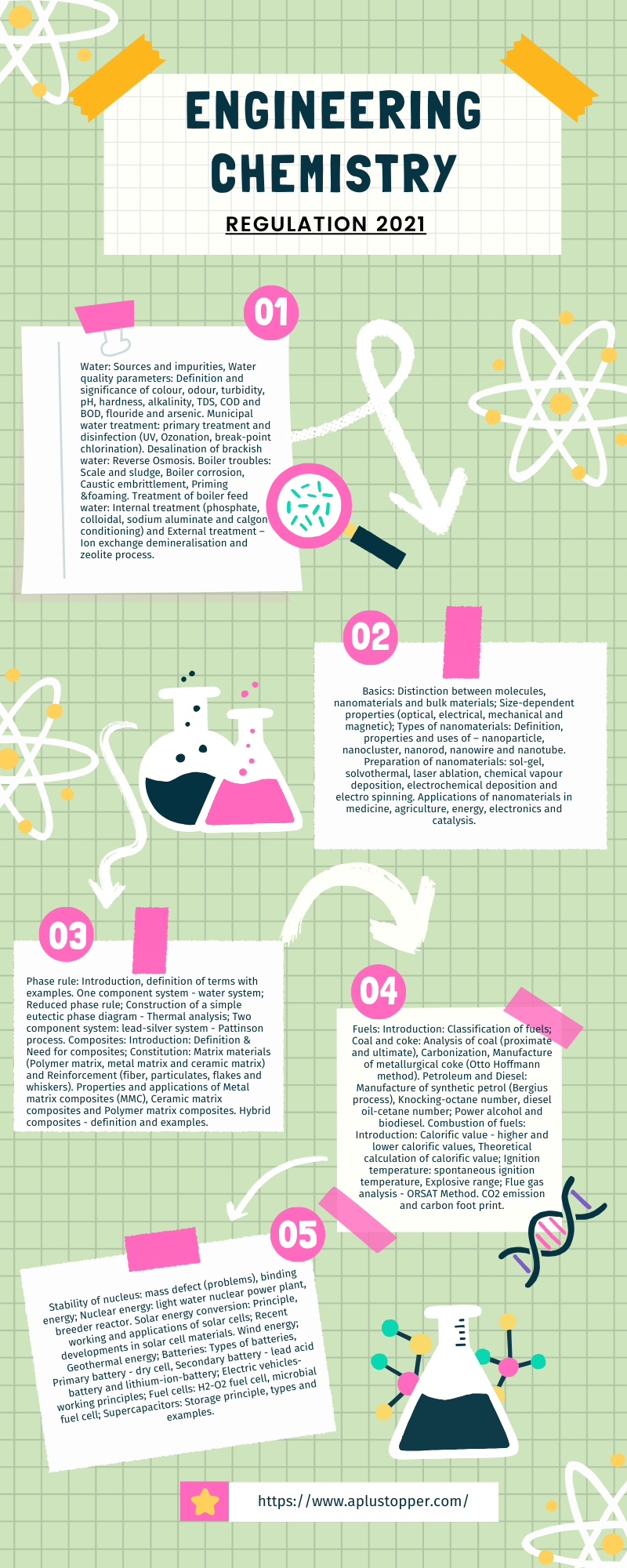
Unit IV: Fuels And Combustion
Fuels: Introduction: Classification of fuels; Coal and coke: Analysis of coal (proximate and ultimate), Carbonization, Manufacture of metallurgical coke (Otto Hoffmann method). Petroleum and Diesel: Manufacture of synthetic petrol (Bergius process), Knocking-octane number, diesel oil-cetane number; Power alcohol and biodiesel. Combustion of fuels: Introduction: Calorific value – higher and lower calorific values, Theoretical calculation of calorific value; Ignition temperature: spontaneous ignition temperature, Explosive range; Flue gas analysis – ORSAT Method. CO2 emission and carbon foot print.
Unit V: Energy Sources And Storage Devices
Stability of nucleus: mass defect (problems), binding energy; Nuclear energy: light water nuclear power plant, breeder reactor. Solar energy conversion: Principle, working and applications of solar cells; Recent developments in solar cell materials. Wind energy; Geothermal energy; Batteries: Types of batteries, Primary battery – dry cell, Secondary battery – lead acid battery and lithium-ion-battery; Electric vehicles-working principles; Fuel cells: H2-O2 fuel cell, microbial fuel cell; Supercapacitors: Storage principle, types and examples.
Text Books:
- P. C. Jain and Monica Jain, “Engineering Chemistry”, 17th Edition, DhanpatRai Publishing Company (P) Ltd, New Delhi, 2018.
- Sivasankar B., “Engineering Chemistry”, Tata McGraw-Hill Publishing Company Ltd, New Delhi, 2008.
- S.S. Dara, “A text book of Engineering Chemistry”, S. Chand Publishing, 12th Edition, 2018.
References:
- B. S. Murty, P. Shankar, Baldev Raj, B. B. Rath and James Murday, “Text book of nanoscience and nanotechnology”, Universities Press-IIM Series in Metallurgy and Materials Science, 2018.
- O.G. Palanna, “Engineering Chemistry” McGraw Hill Education (India) Private Limited, 2nd Edition, 2017.
- Friedrich Emich, “Engineering Chemistry”, Scientific International PVT, LTD, New Delhi, 2014.
- Shikha Agarwal, “Engineering Chemistry-Fundamentals and Applications”, Cambridge University Press, Delhi, Second Edition, 2019.
- O.V. Roussak and H.D. Gesser, Applied Chemistry-A Text Book for Engineers and Technologists, Springer Science Business Media, New York, 2nd Edition, 2013.
Get details of other branches which are providing by the anna university under four year undergraduate programme connected to affiliated institutions in detailed way in this link, Anna University Regulation 2021.
Related Posts Of Semester – I:
- HS3152 – Professional English I Syllabus
- MA3151 – Matrices And Calculus Syllabus
- PH3151 – Engineering Physics Syllabus
