What is Covalent Bond?
The Covalent Bond
- Covalent bonds are formed when atoms of non-metals combine with each other to form a molecule.
Non-metal + Non-metal → Covalent compound - The non-metals involved can be
(a) the elements from Groups 15, 16, and 17 of the Periodic Table.
(b) carbon and silicon from Group 14 of the Periodic Table.
(c) hydrogen, the smallest-sized atom. Hydrogen forms covalent bonds when it combines with more electronegative non-metal such as a fluorine, oxygen, nitrogen, chlorine, bromine, iodine or carbon. - During the formation of covalent bonds, atoms of non-metals share electrons to achieve stable noble gas electron arrangements.
- Each atom contributes the same number of electrons to each other for sharing.
- The shared pairs of electrons which bind the atoms together are called covalent bonds.
As a result, covalent molecules are formed. - The chemical bond formed when two atoms share electrons between them is known as a covalent bond.
- The sharing of electrons between the two atoms takes place in such a way that both the atoms acquire the stable electronic configurations of their nearest noble gases.
Types of Covalent Bonds
- During the formation of a covalent bond between two atoms, each atom contributes 1, 2 or 3 electrons to each other for sharing.
- By doing so, the two atoms share 1, 2 or 3 pairs of electrons so as to achieve stable noble gas electron arrangements.
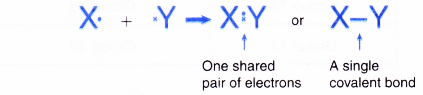
- When two atoms share one pair of electrons, a single covalent bond is formed.
- When two atoms share two pairs of electrons, a double covalent bond is formed.
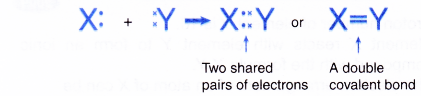
- When two atoms share three pairs of electrons, a triple covalent bond is formed.
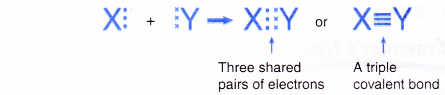
- Hence, there are three types of covalent bonds:
(a) Single covalent bond
(b) Double covalent bond
(c) Triple covalent bond - The number of electrons contributed by an atom of an element for sharing during the formation of covalent bonds is shown below.
- An atom of a Group 17 element contributes one electron for sharing because it has 7 valence electrons.
- An atom of a Group 16 element contributes two electrons for sharing because it has 6 valence electrons.
- An atom of a Group 15 element contributes three electrons for sharing because it has 5 valence electrons.
- A silicon or carbon atom from Group 14 contributes four electrons for sharing because both these elements have four valence electrons.
- A hydrogen atom contributes one electron for sharing because it has one valence electron.
People also ask
- Chemical Bonding and Compound Formation
- Chemical Bonding
- How is covalent bond is formed?
- Describe how to write a formula for a covalent compound
- What causes ions to form ionic bonds?
- Explain the formation of ionic bonds with examples
- Properties of Ionic and Covalent Compounds
- How do you write the formula for ionic compounds?
- How do you Name an Ionic Compound?
1. Single covalent bond:
A single covalent bond is formed when one pair of electrons is shared between two atoms.
Examples :
1. Formation of a hydrogen molecule (H2) :
A molecule of hydrogen consists of two hydrogen atoms. Each hydrogen atom has one electron. When two atoms of hydrogen combine, one electron of each takes part in sharing. Thus, two electrons (one pair of electrons) are shared between the two atoms.
![]() The shared electron pair always exists between the two atoms. The two dots between the two H atoms represent the pair of shared electrons. One pair of shared electrons gives a single bond. Such a bond is represented by a short line between the two atoms. Thus, a hydrogen molecule may be represented as in figure.
The shared electron pair always exists between the two atoms. The two dots between the two H atoms represent the pair of shared electrons. One pair of shared electrons gives a single bond. Such a bond is represented by a short line between the two atoms. Thus, a hydrogen molecule may be represented as in figure.
![]() Once the bond is formed, the both atoms have a stable configuration of the noble gas helium.
Once the bond is formed, the both atoms have a stable configuration of the noble gas helium.
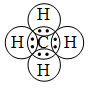
2. Formation of a methane molecule (CH4) :
A carbon atom has four electrons in its outermost shell (valence shell). It shares its valence electrons with those of four H atoms. Thus, an atom of carbon forms four single covalent bonds with four H atoms.
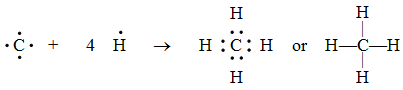 Pictorially, a methane molecule may be represented as in Figure.
Pictorially, a methane molecule may be represented as in Figure.
 2. Double covalent bond :
2. Double covalent bond :
A double covalent bond is formed when two pairs of electrons are shared between the two combining atoms. A sharing of two pairs of electrons is shown by marking two short lines between the symbols of the two atoms.
Examples:
1. Formation of an oxygen molecule (O2) :
An atom of oxygen contains six electrons in its valence shell. It requires two more electrons to attain a stable eight-electron configuration (octet). This is achieved when each of the two oxygen atoms shares its two electrons with the other, resulting in the formation of a stable oxygen molecule.
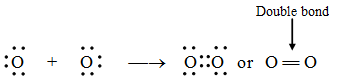 Pictorially, the oxygen molecule may be represented as in figure.
Pictorially, the oxygen molecule may be represented as in figure.
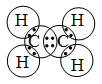
![]() 2. Formation of an ethylene molecule (C2H4) :
2. Formation of an ethylene molecule (C2H4) :
In the formation of an ethylene molecule (C2H4), each of the two C atoms combines with two H atoms to form two single covalent bonds. The remaining two electrons of each C atom form a double bond between the two C atoms.
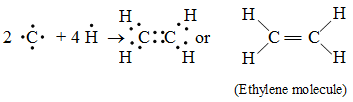 Pictorially, a molecule of ethylene (C2H4) may be represented a in figure.
Pictorially, a molecule of ethylene (C2H4) may be represented a in figure.
 3. Triple covalent bond:
3. Triple covalent bond:
A triple covalent bond is formed when three pairs of electrons (six electrons) are shared between the two combining atoms. A triple bond is shown by marking three short lines between the two symbols of the atoms.
Examples:
1. Formation of a nitrogen molecule (N2) :
An atom of nitrogen has five electrons in its valence shell. It requires three more electrons to attain the stable octet. This is achieved when two nitrogen atoms combine together by sharing three electrons each to form a nitrogen molecule.
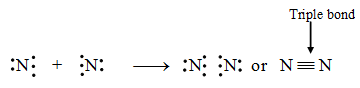 Pictorially, a nitrogen molecule can be represented as in figure.
Pictorially, a nitrogen molecule can be represented as in figure.
![]() 2. Formation of an acetylene molecule (C2H2) :
2. Formation of an acetylene molecule (C2H2) :
In an acetylene molecule, two C atoms combine with two H atoms. Each C atom shares three of its valence electrons with the other C atom. One electron of each C atom is shared with one electron of a H atom.
![]() Thus, in a molecule of acetylene, there is a triple covalent bond between the two C atoms and each C atom is joined to one H atom by a single covalent bond. Pictorially, a molecule of acetylene may be represented as in figure.
Thus, in a molecule of acetylene, there is a triple covalent bond between the two C atoms and each C atom is joined to one H atom by a single covalent bond. Pictorially, a molecule of acetylene may be represented as in figure.
Characteristics of covalent compounds:
- Covalent compounds are made up of neutral molecules. Hence, the forces of attraction between the molecules are weaker than those found in ionic compounds. Therefore, covalent compounds are usually volatile liquids or gases.
- The melting and the boiling points of covalent compounds are generally low. Since covalent compounds are made up of neutral molecules, the forces of attraction between the molecules are very weak. So, a comparatively small amount of heat energy is required to break these weak intermolecular forces of attraction. Hence, they have low melting and boiling points.
- Covalent compounds are insoluble in water but soluble in organic solvents.
- Covalent compounds do not conduct electricity. This is because they are made up of neutral molecules, not ions, and do not produce ions in the molten state or in aqueous solutions.
Bonding in metals:
As you know, metals are hard solids and they are made up of atoms. It has been established that the atoms in a metal are very closely packed together.
The force that holds the atoms closely together in a metal is known as the metallic bond.
Metal atoms lose one, two or three electrons to form positively charged ions, called cations.
The electrons thus lost move freely in the metal, i.e., these electrons become mobile, but the cations do not leave their positions. So in a metal lattice it is assumed that the metal ions are immersed in a sea of electrons. Due to the presence of mobile electrons, metals are good conductors of heat and electricity.
