Constructing ionic equations using the continuous variation method
- The ionic equation for the formation of an insoluble salt can be constructed if we know the number of moles of cation and anion reacted together to form 1 mole of the insoluble salt.
- For example:
(a) 1 mole of silver chromate(VI) is formed from 2 moles of Ag+ ions and 1 mole of CrO42- ions.
Ionic equation:
2Ag+(aq) + CrO42- (aq) → Ag2CrO4 (s)
(b) 1 mole of lead(II) bromide is formed from 1 mole of Pb2+ ions and 2 moles of Br ions. Ionic equation:
Pb2+(aq) + 2Br–(aq) → PbBr2(s) - The number of moles of cation and anion which combine to form 1 mole of the insoluble salt can be determined from experiment by a continuous variation method.
- The method involves the following steps.
- Carry out a reaction between a fixed volume of reactant A with varying volumes of a second reactant B.
- Determine the volume of reactant B required to react completely with the fixed volume of reactant A.
- Use the results of the experiment to calculate the number of moles of reactant A and number of moles of reactant B which reacted completely with each other.
- Determine the simplest mole ratio of reactant A to reactant B which combine to form one mole of the insoluble salt.
- Use the ratio to construct the ionic equation.
Constructing ionic equations example
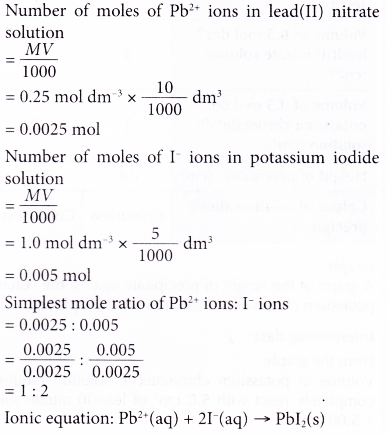
1. 10 cm3 of 0.25 mol dm-3 lead(II) nitrate solution reacts completely with 5 cm3 of 1.0 mol dm-3 potassium iodide solution. A yellow precipitate of lead(II) iodide is formed. Construct the ionic equation for the formation of lead(II) iodide.
Solution:
People also ask
- Classification of Salts
- General Properties of Salts
- Uses of different salts in daily life
- Preparation of Salts
- Describe the preparation of soluble and insoluble salts
- Qualitative Analysis of Salts
- Action of Heat on Salts
- Test for Cations and Anions in Aqueous Solutions
- What is stoichiometry and why is it used in chemistry?
Constructing ionic equations experiment
Aim: To construct an ionic equation for the formation of lead(II) chromate(VI).
Problem statement: How to construct an ionic equation for the formation of lead(II) chromate(VI)?
Hypothesis: As the volume of potassium chromate(VI) solution increases, the height of the yellow precipitate increases until all the lead(II) nitrate has reacted.
Variables:
(a) Manipulated variable : Volume of potassium chromate(VI) solution
(b) Responding variable : Height of yellow lead(II) chromate(VI) precipitate
(c) Controlled variables : Volume and concentration of lead(II) nitrate solution, concentration of potassium chromate(VI) solution, size of test tubes
Materials: 0.5 mol dm-3 lead(II) nitrate solution and 0.5 mol dm-3 potassium chromate(VI) solution.
Apparatus: 7 test tubes, test tube rack, burettes, metre rule, stopper, dropper, retort stand and clamp.
Procedure:
- Seven test tubes of the same size are labelled from 1 to 7 and are placed in a test tube rack.
- A burette is filled with 0.5 mol dm-3 lead(II) nitrate solution. 5.00 cm3 of the lead(II) nitrate solution is added to each of the seven test tubes.
- A second burette is filled with 0.5 mol dm-3 potassium chromate(VI) solution. The potassium chromate(VI) solution is added to each of the seven test tubes according to the volumes specified in Table.
- Each test tube is stoppered and shaken well. The test tubes are left aside for about half an hour to allow the precipitate to settle.
- The height of precipitate in each test tube is measured. The colour of the solution above the precipitate in each test tube is noted.
- All readings and observations are recorded in Table.
Results:
| Test tube | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Volume of 0.5 mol dm-3 lead(II) nitrate solution | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Volume of 0.5 mol dm-3 potassium chromate(VI) solution (cm3) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Height of precipitate (cm) | 0.6 | 1.2 | 1.8 | 2.4 | 3.0 | 3.0 | 3.0 |
| Colour of solution above precipitate | Colourless | Colourless | Colourless | Colourless | Colourless | Yellow | Yellow |
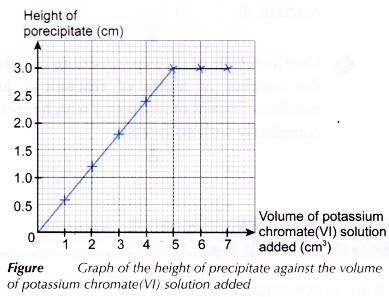
Graph:
A graph of the height of precipitate against the volume of potassium chromate(VI) solution added is plotted.
 Interpreting data:
Interpreting data:
From the graph:
Volume of potassium chromate(VI) solution required to completely react with 5.0 cm3 of lead(II) nitrate solution = 5.00 cm3

Number of moles of Pb2+ ions in 5.00 cm3 of 0.5 mol dm-3 lead(II) nitrate solution in each of the test tubes
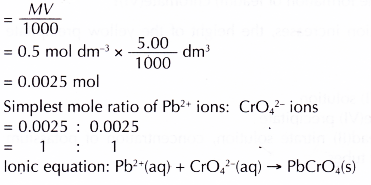
Discussion:
- A yellow precipitate of lead(II) chromate(VI) is formed in each of the seven test tubes.
- The height of the precipitate increases gradually from test tubes 1 to 5 because more and more lead(II) chromate(VI) is formed due to the increasing amount of potassium chromate(VI) added to the test tubes.
- The first test tube to achieve maximum constant height of precipitate indicates a complete reaction has taken place. The volume of potassium chromate(VI) solution (5.00 cm3) added is just sufficient to react completely with the lead(II) nitrate in the test tube.
- The colour of the solution above the precipitate in test tubes 1 to 4 are colourless due to the excess lead(II) nitrate.
- In test tube 5, a complete reaction has taken place. No reactants are present in excess.
- The colour of the solution above the precipitate in test tubes 6 and 7 are yellow due to the excess potassium chromate(VI).
Conclusion:
The ionic equation for the formation of lead(II) chromate(VI) can be obtained from a precipitation reaction. The hypothesis is accepted.
